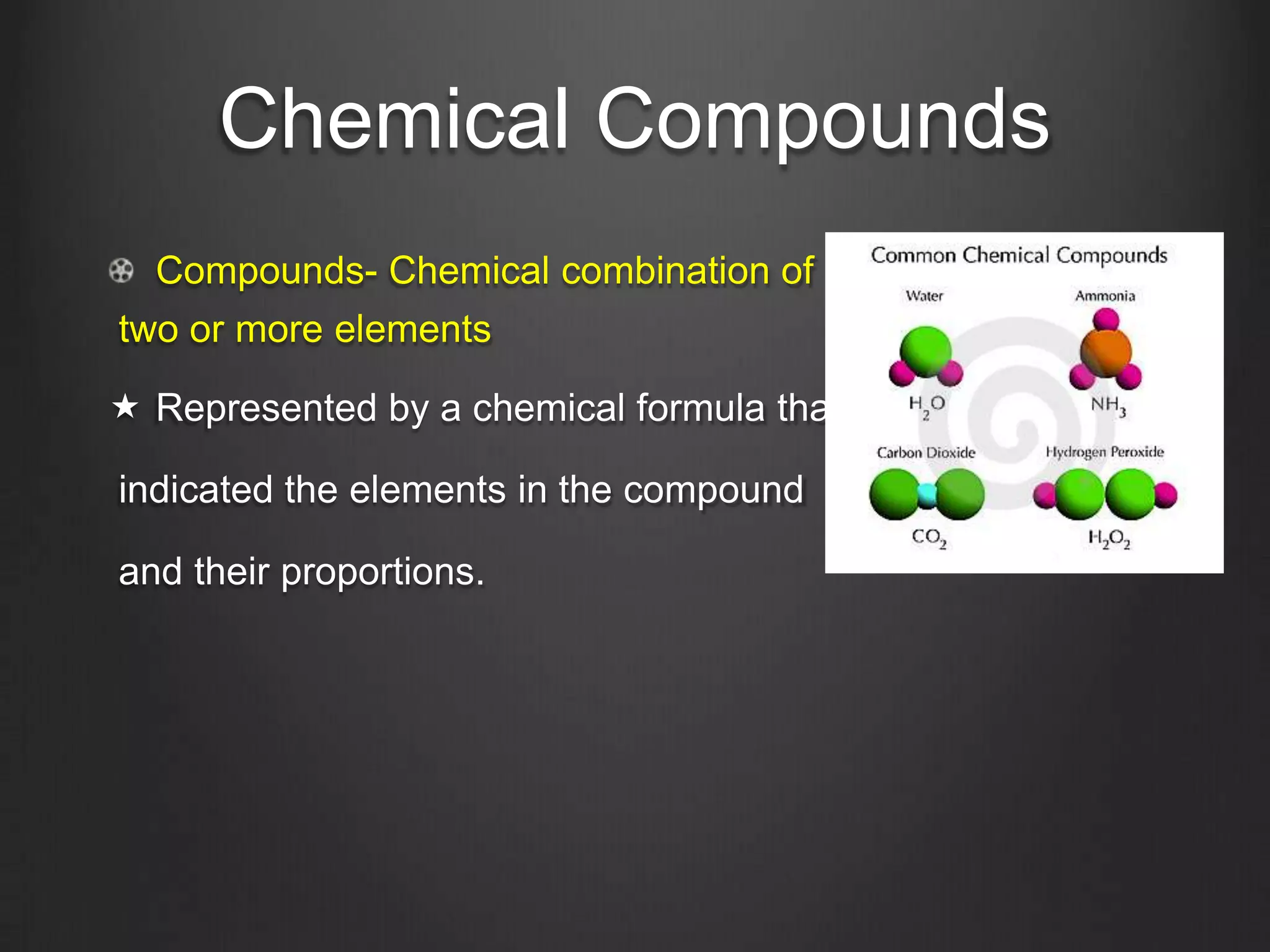
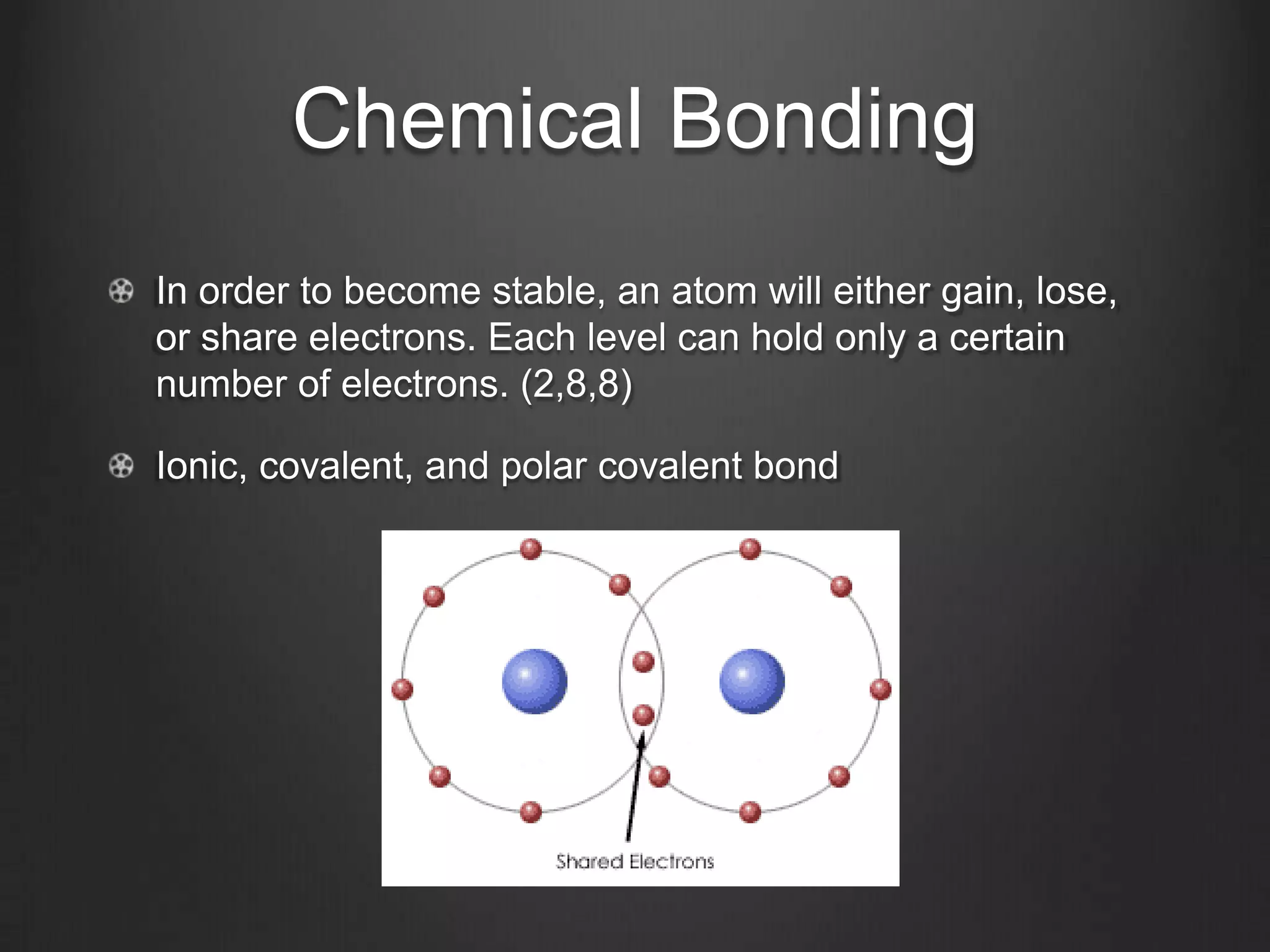
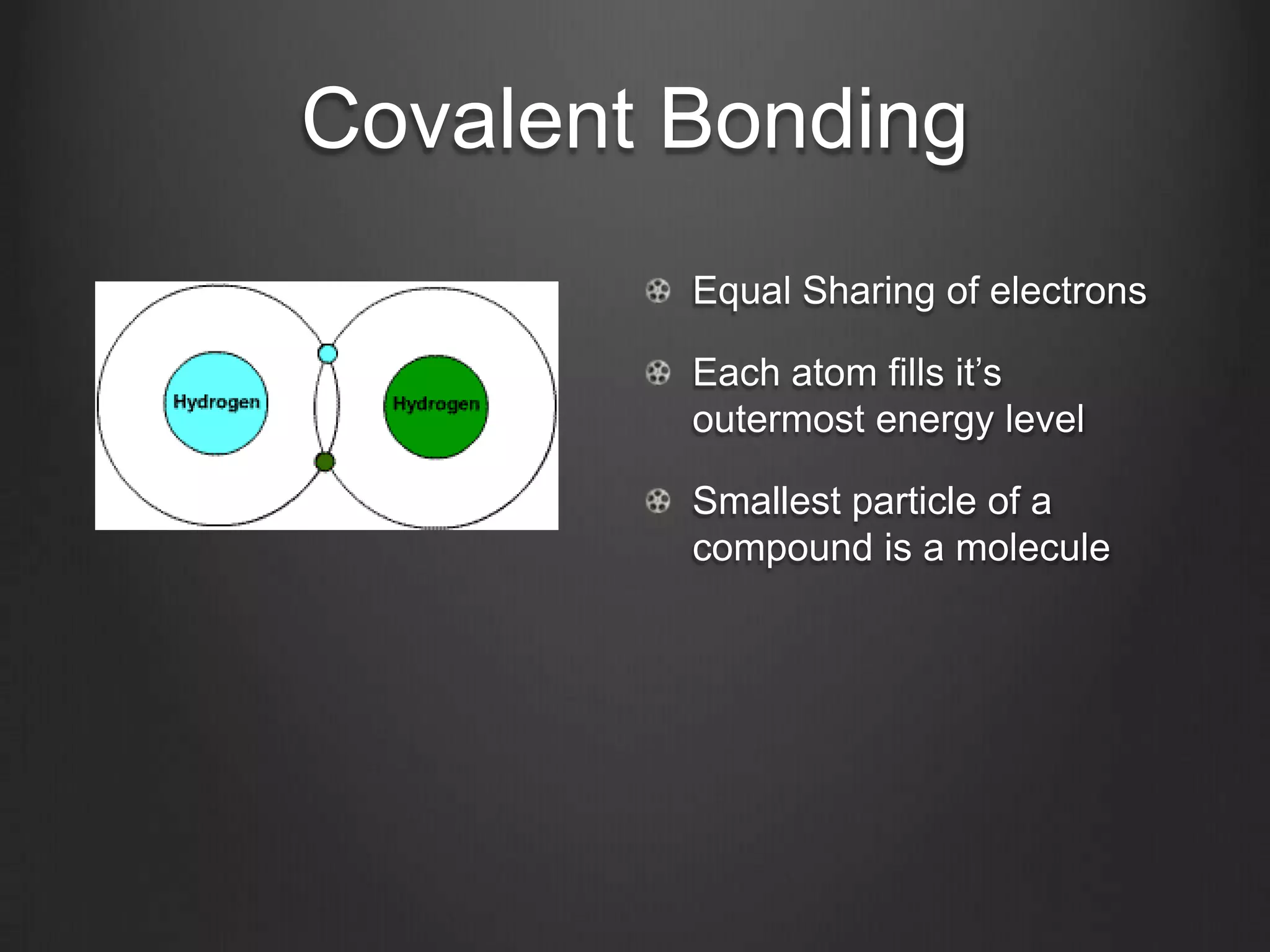
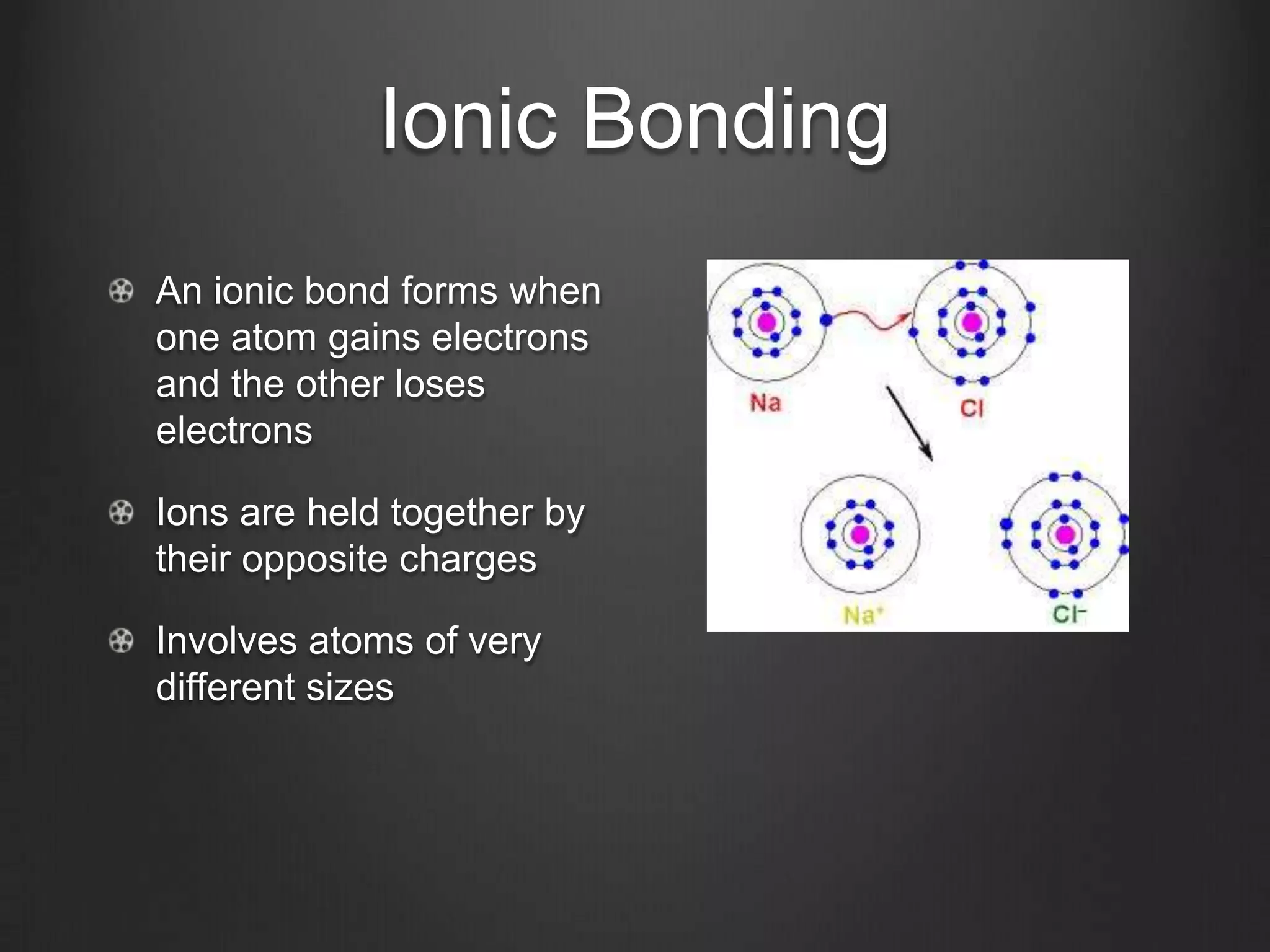
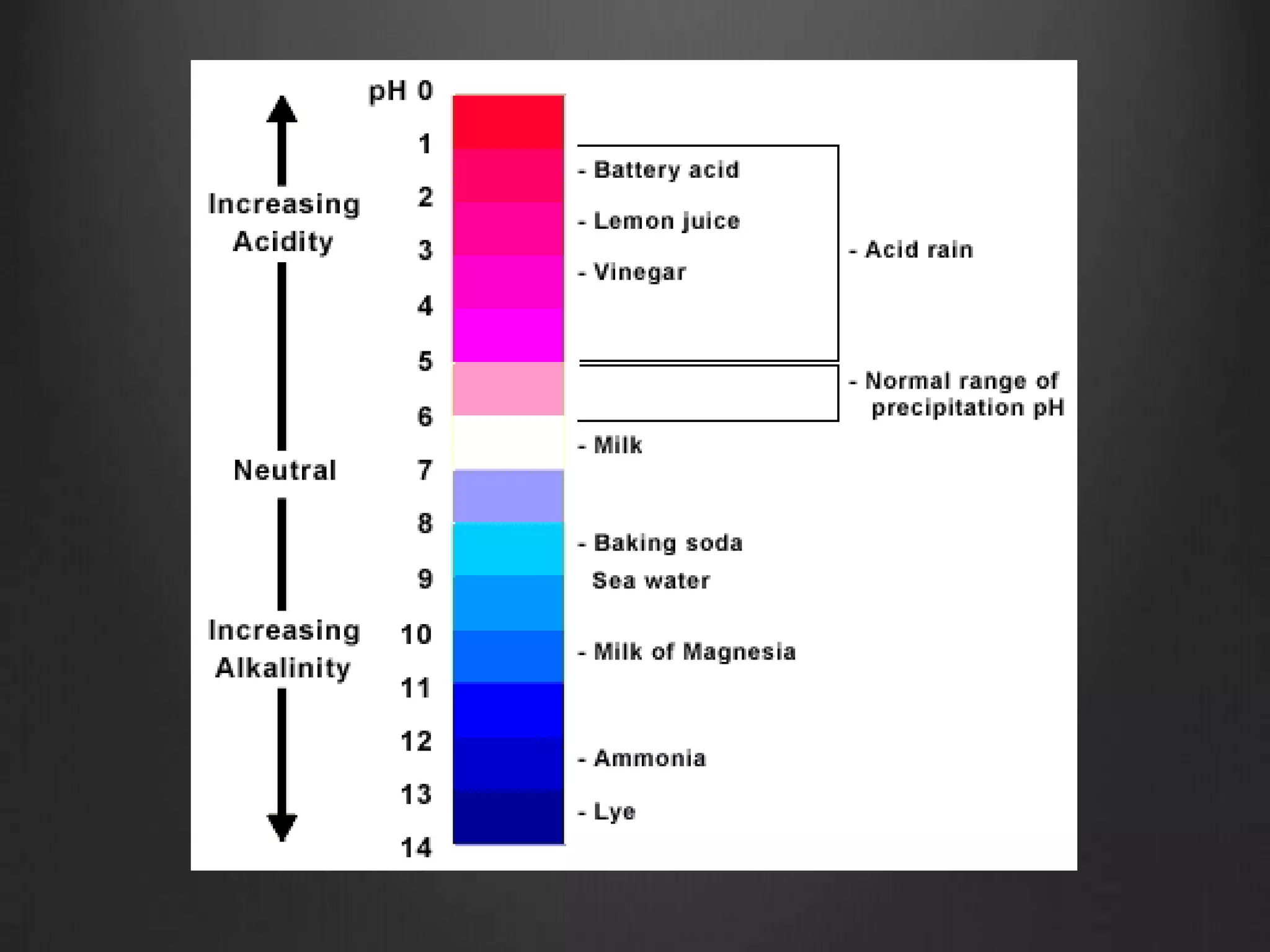
This document discusses key concepts in chemistry including the structure of atoms, elements, compounds, and chemical bonding. It explains that atoms are made up of protons, neutrons, and electrons; that elements are composed of only one type of atom; and that compounds are formed by chemical combinations of two or more elements. The three main types of chemical bonding - ionic, covalent, and polar covalent - are introduced along with examples of how they form compounds. Properties of water related to its chemical structure and hydrogen bonding are also summarized.