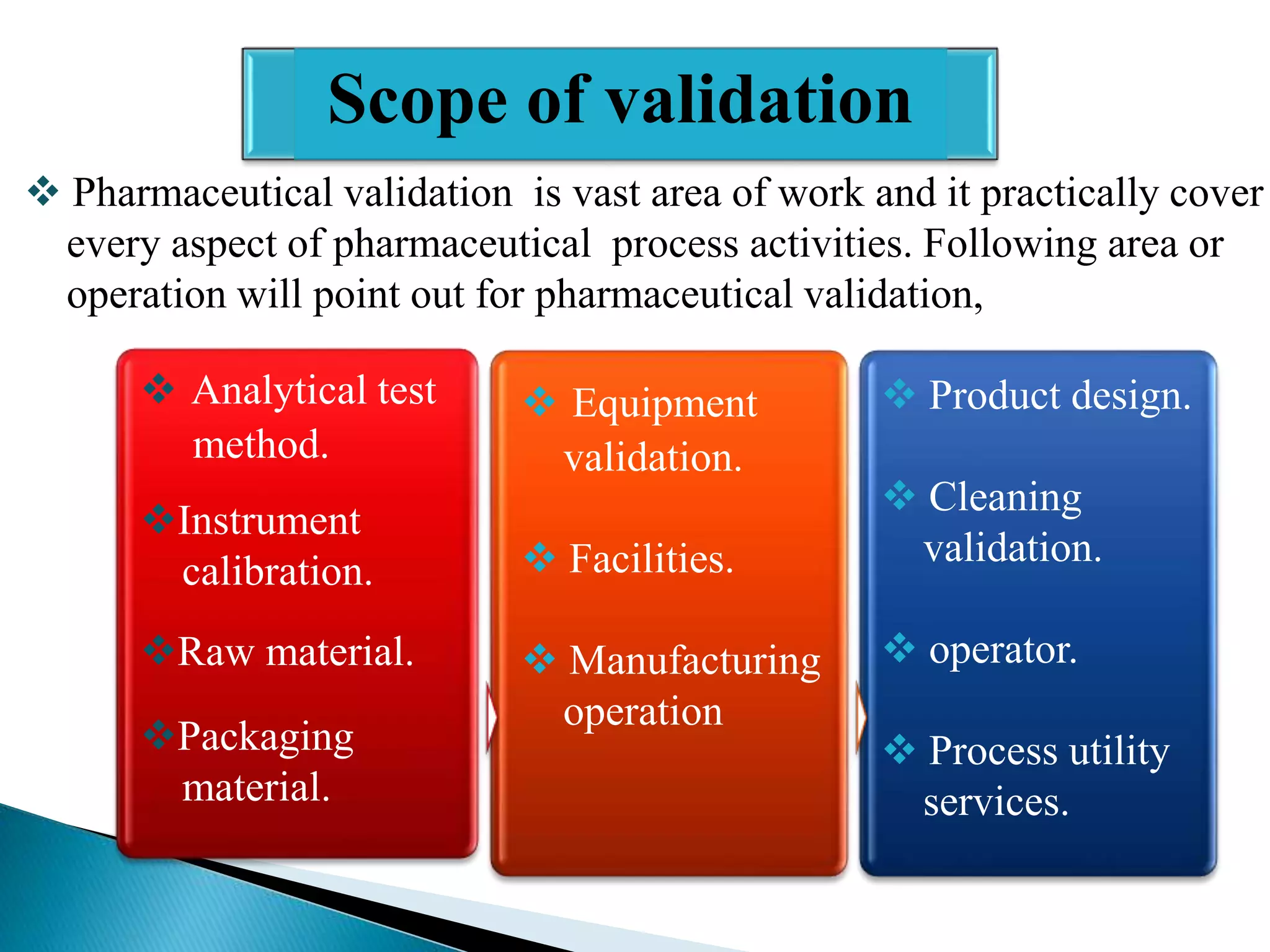
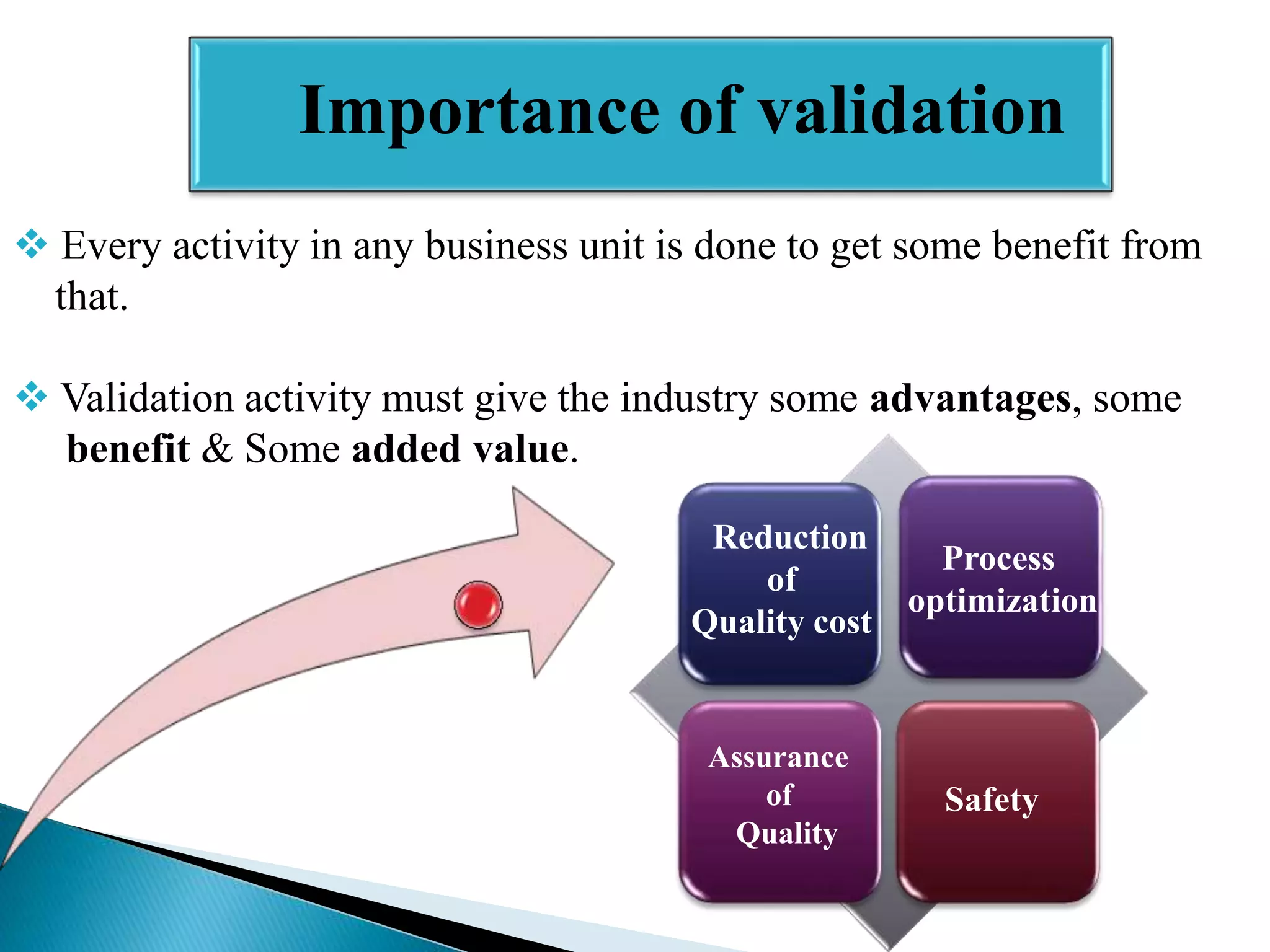
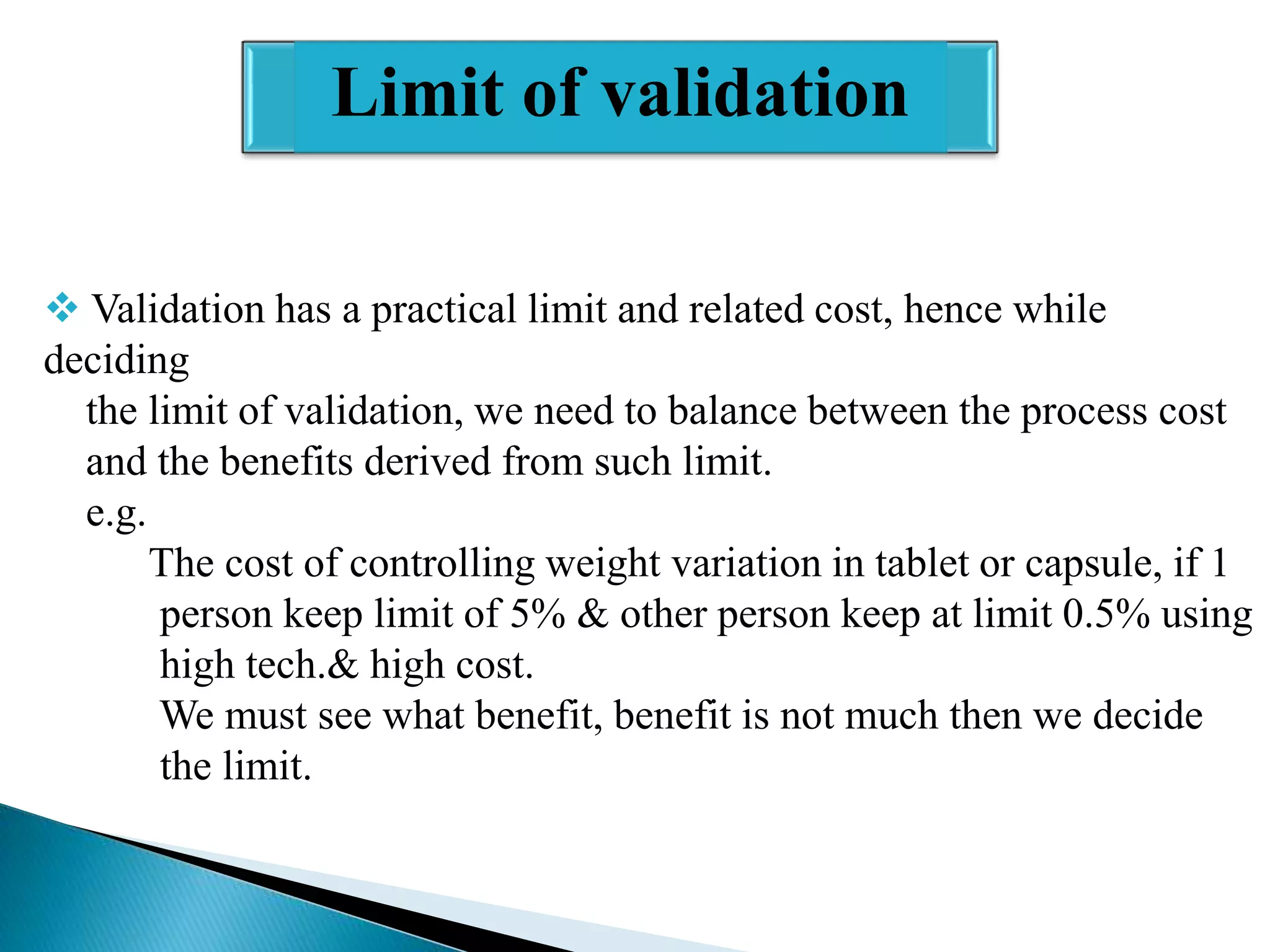
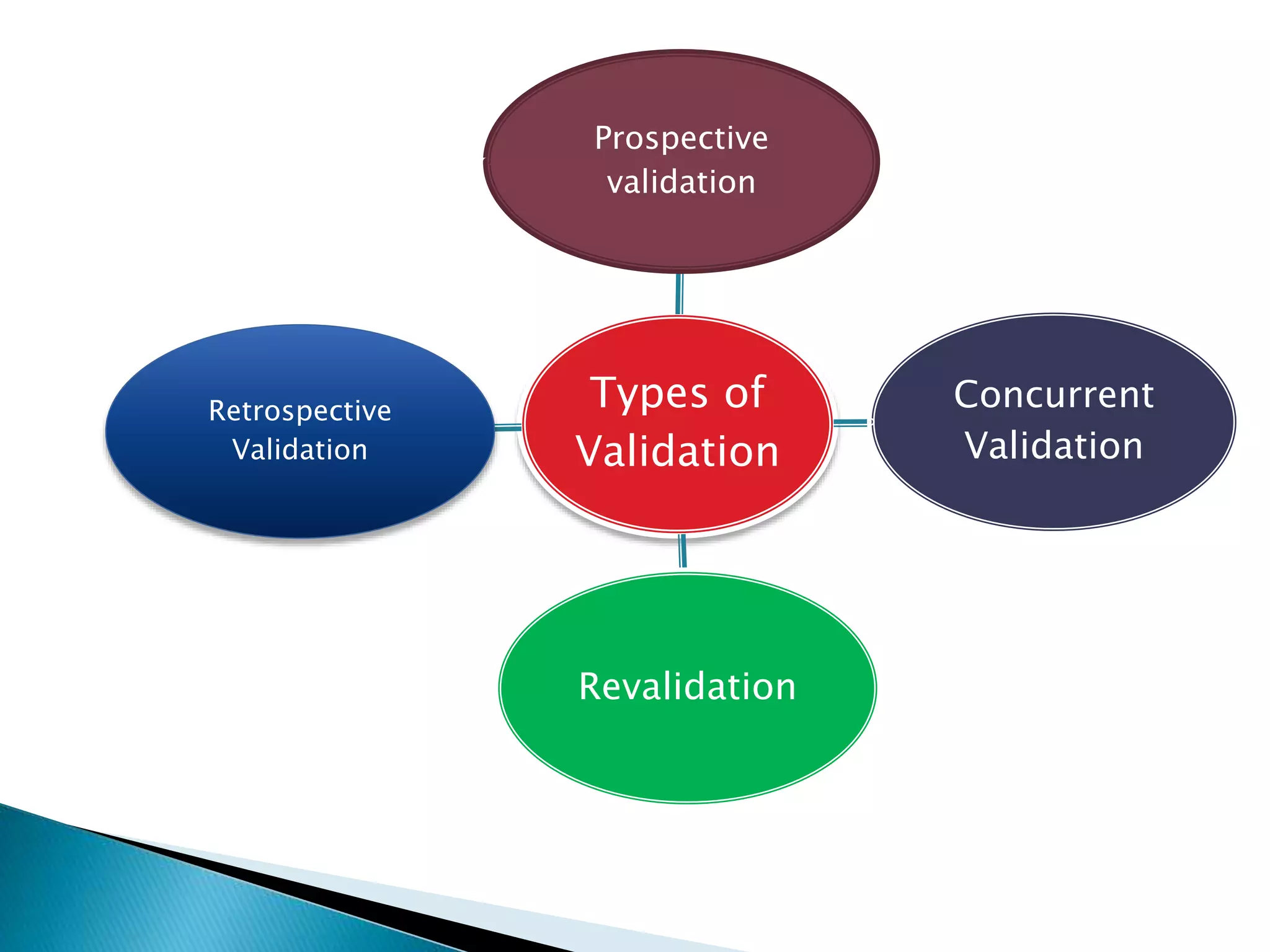
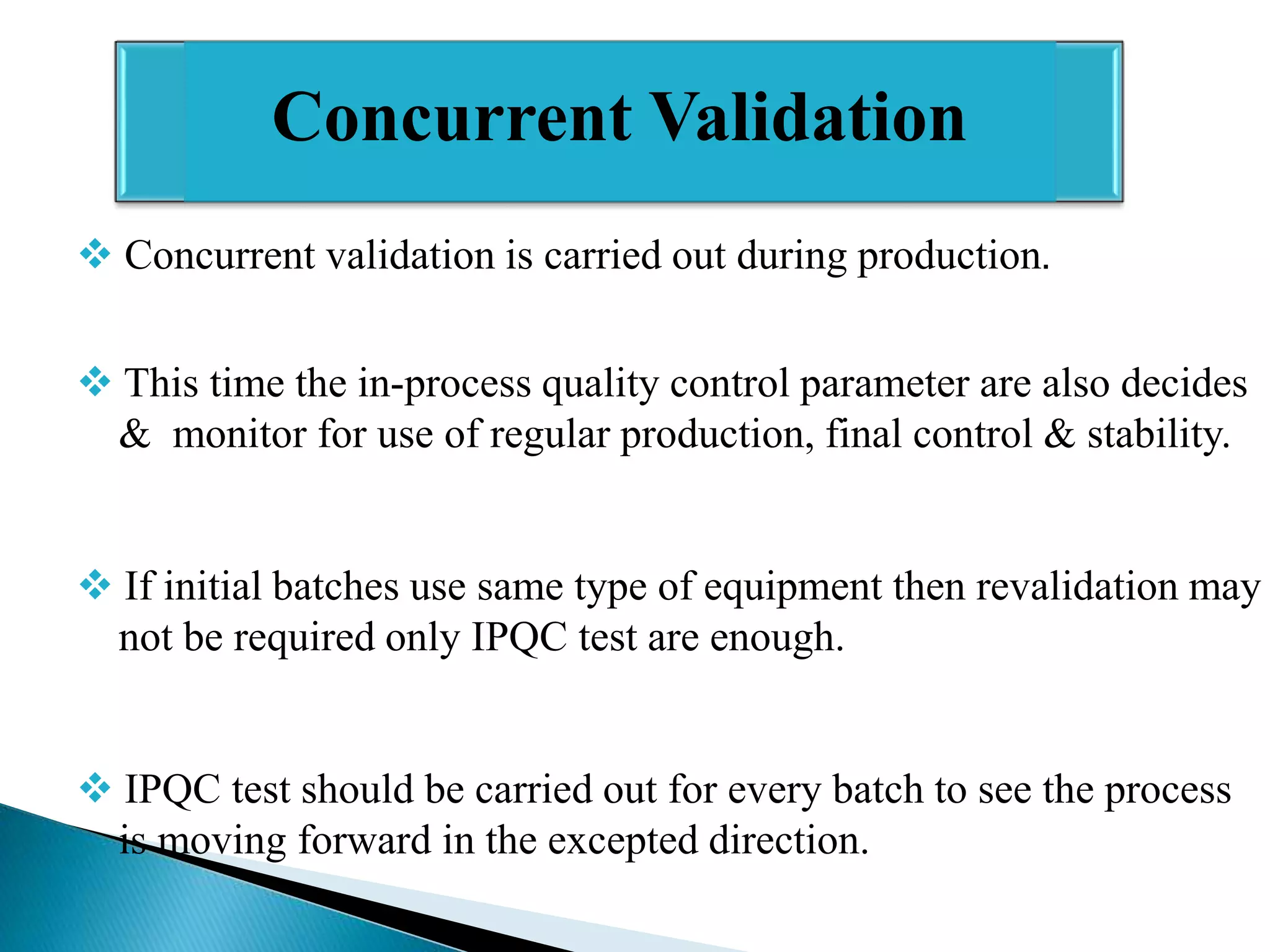
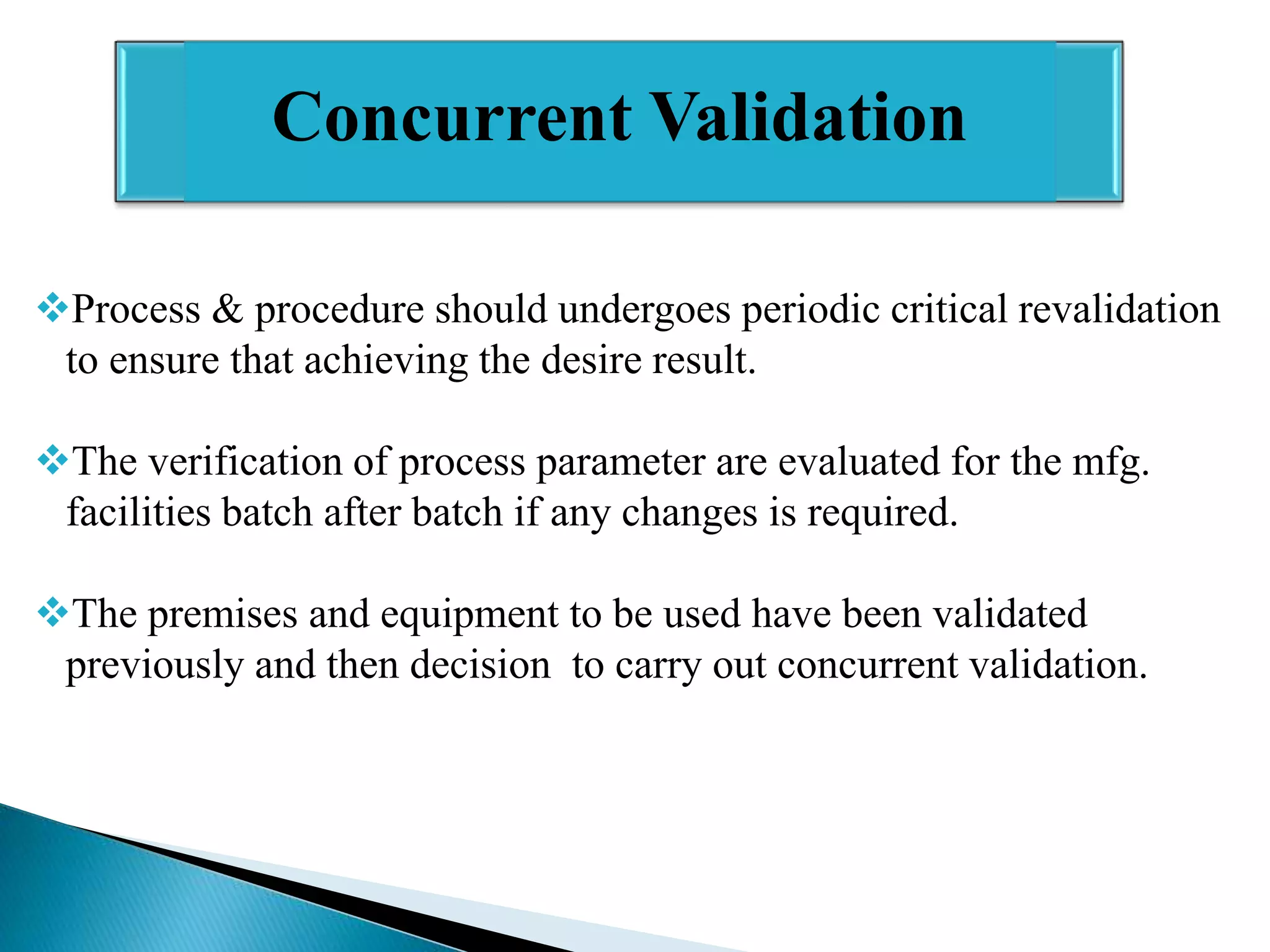
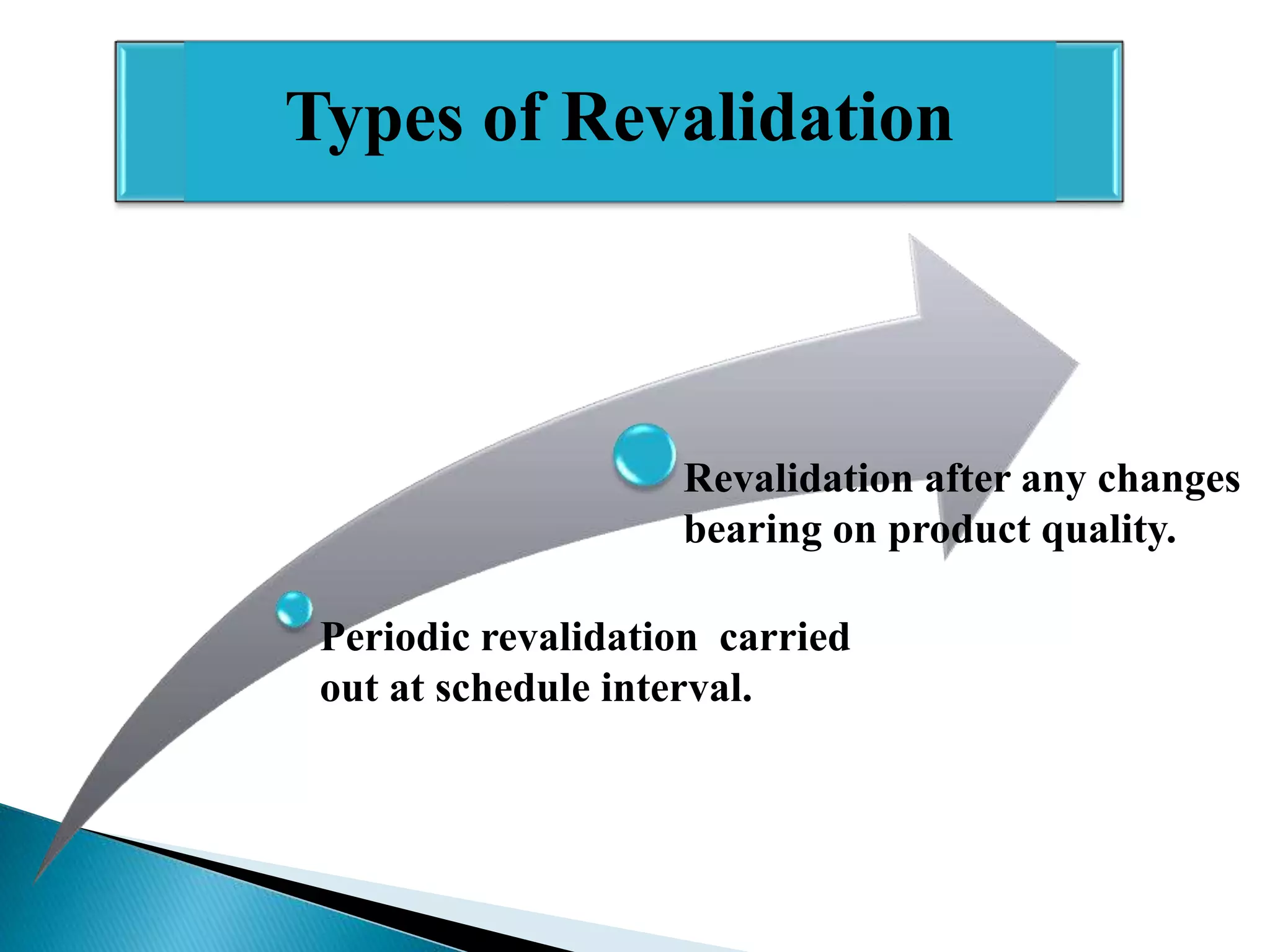
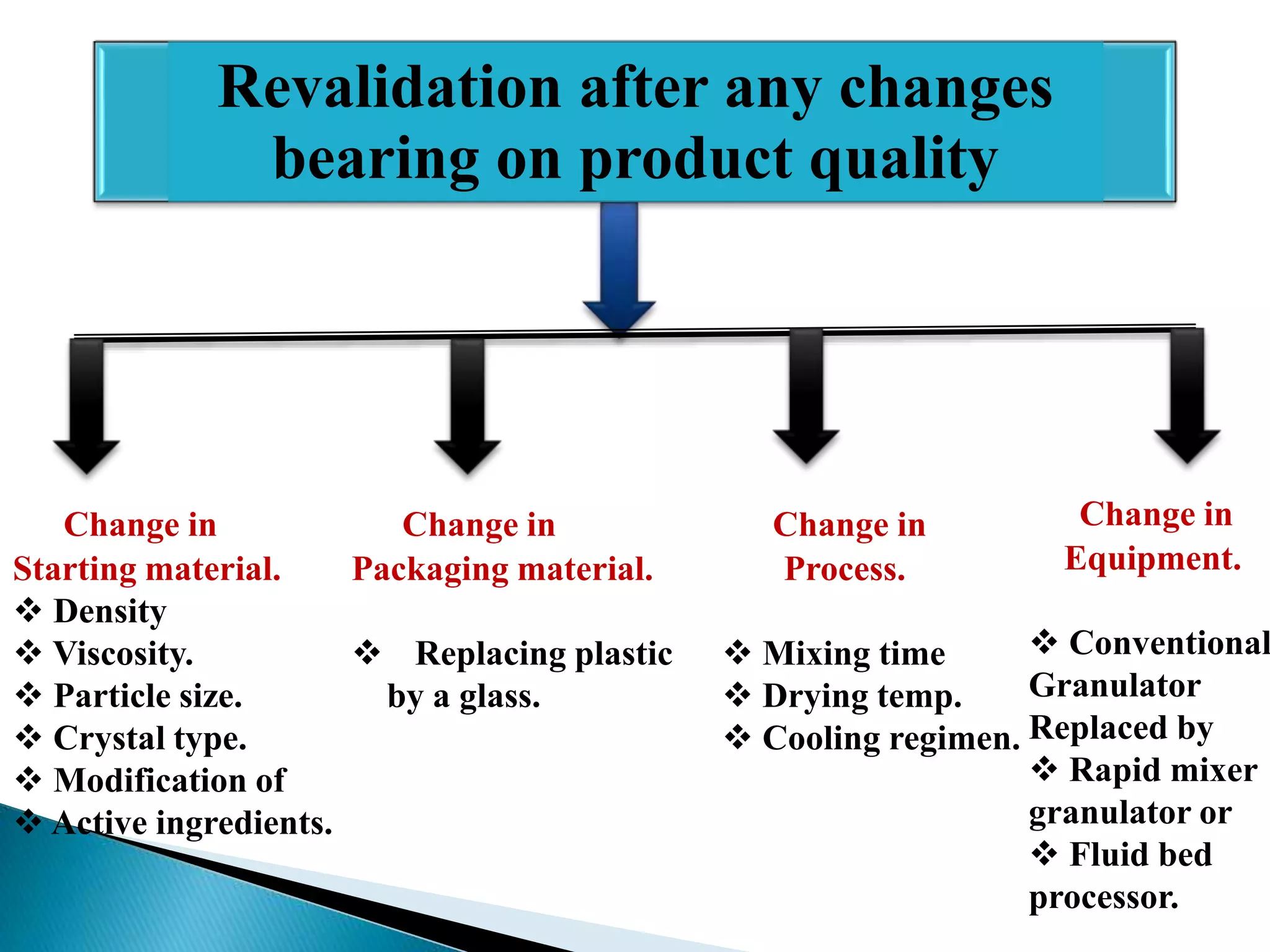
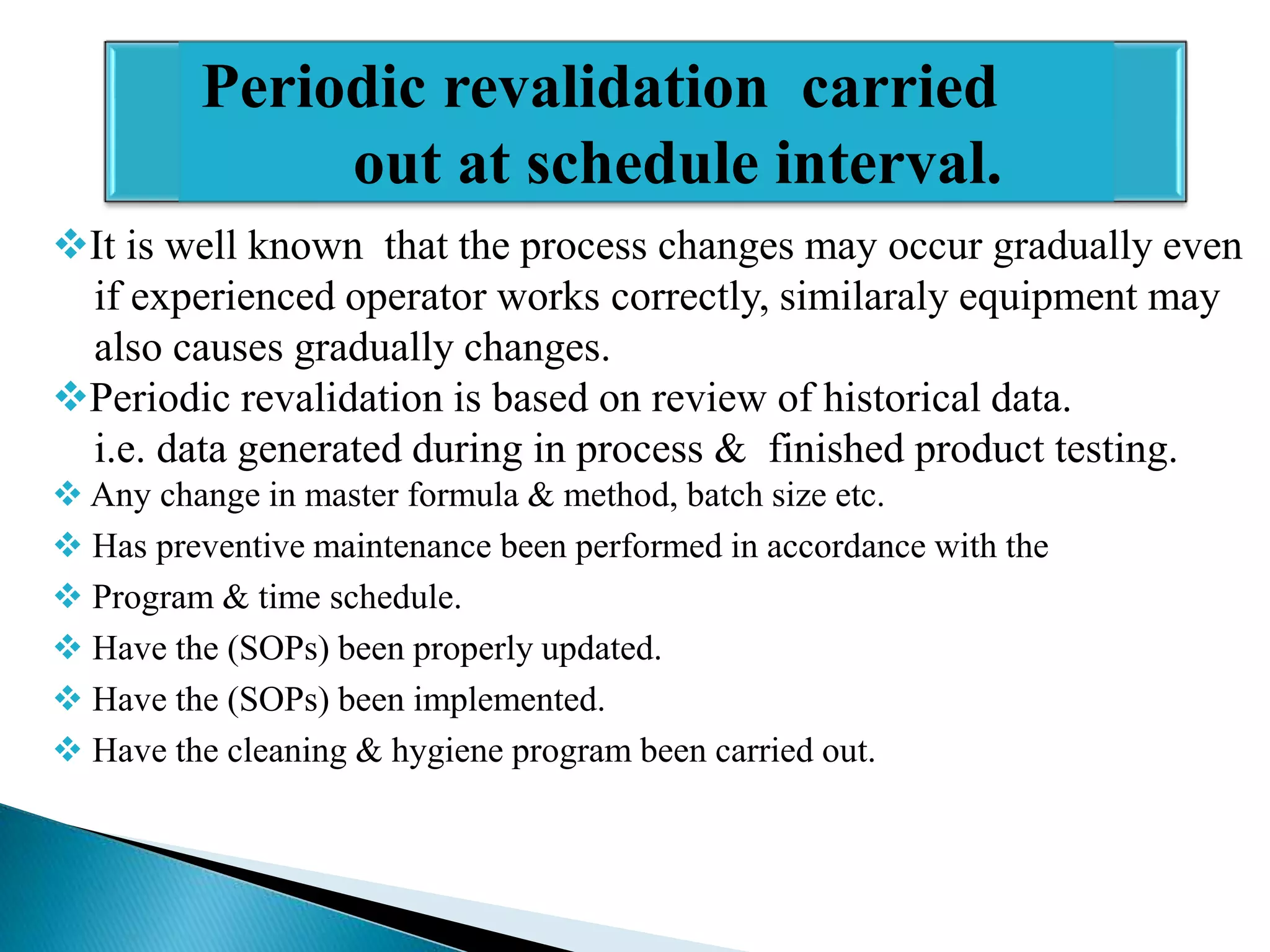
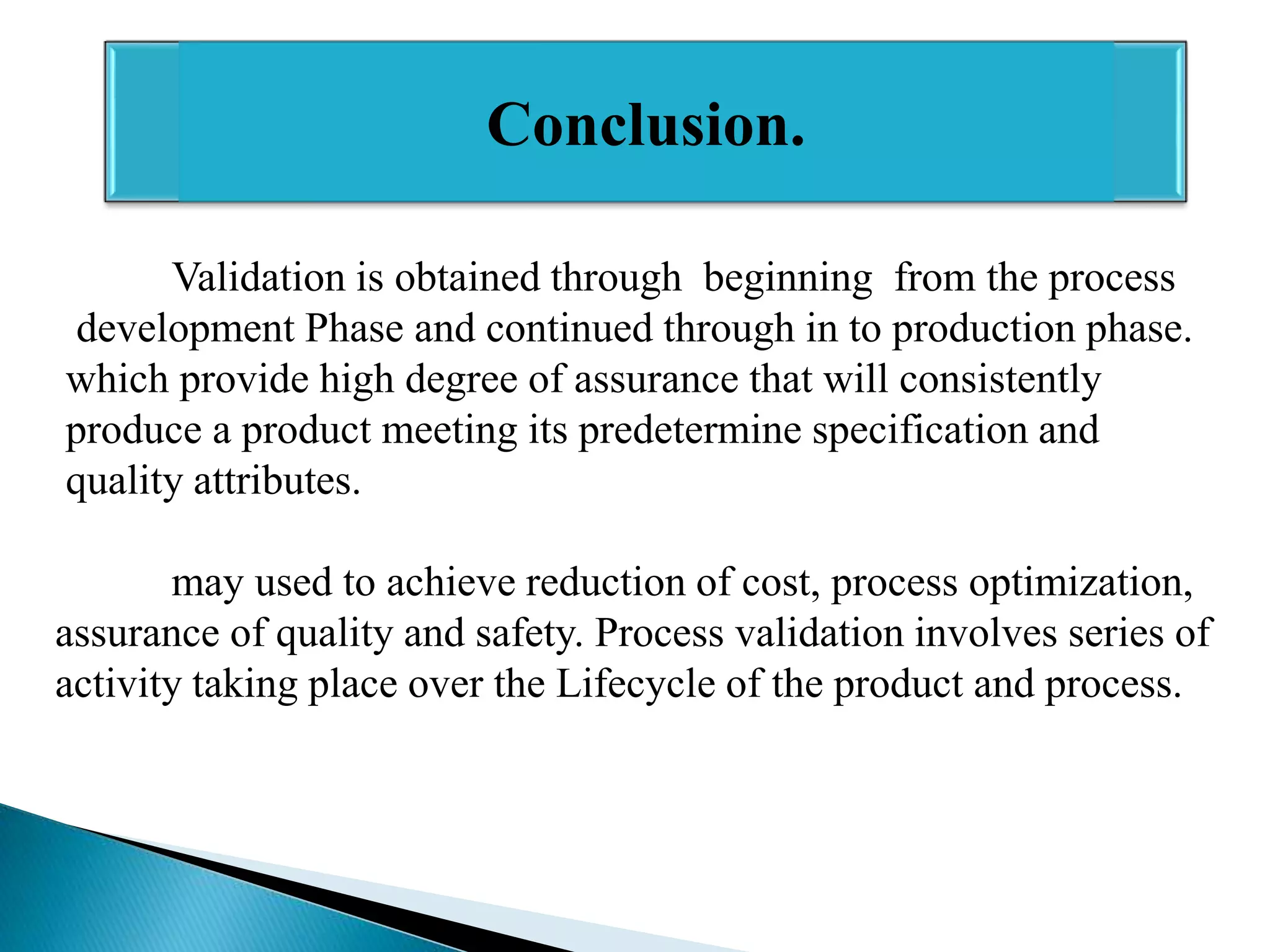
The document outlines the concept and importance of validation in the pharmaceutical industry, emphasizing its necessity for ensuring consistent product quality through a documented program that covers various aspects such as analytical testing and equipment calibration. It discusses process validation, including its phases and types—prospective, concurrent, and retrospective validation—along with the need for revalidation when changes occur. The overall goal of validation is to optimize processes, reduce costs, assure quality, and enhance safety in pharmaceutical production.