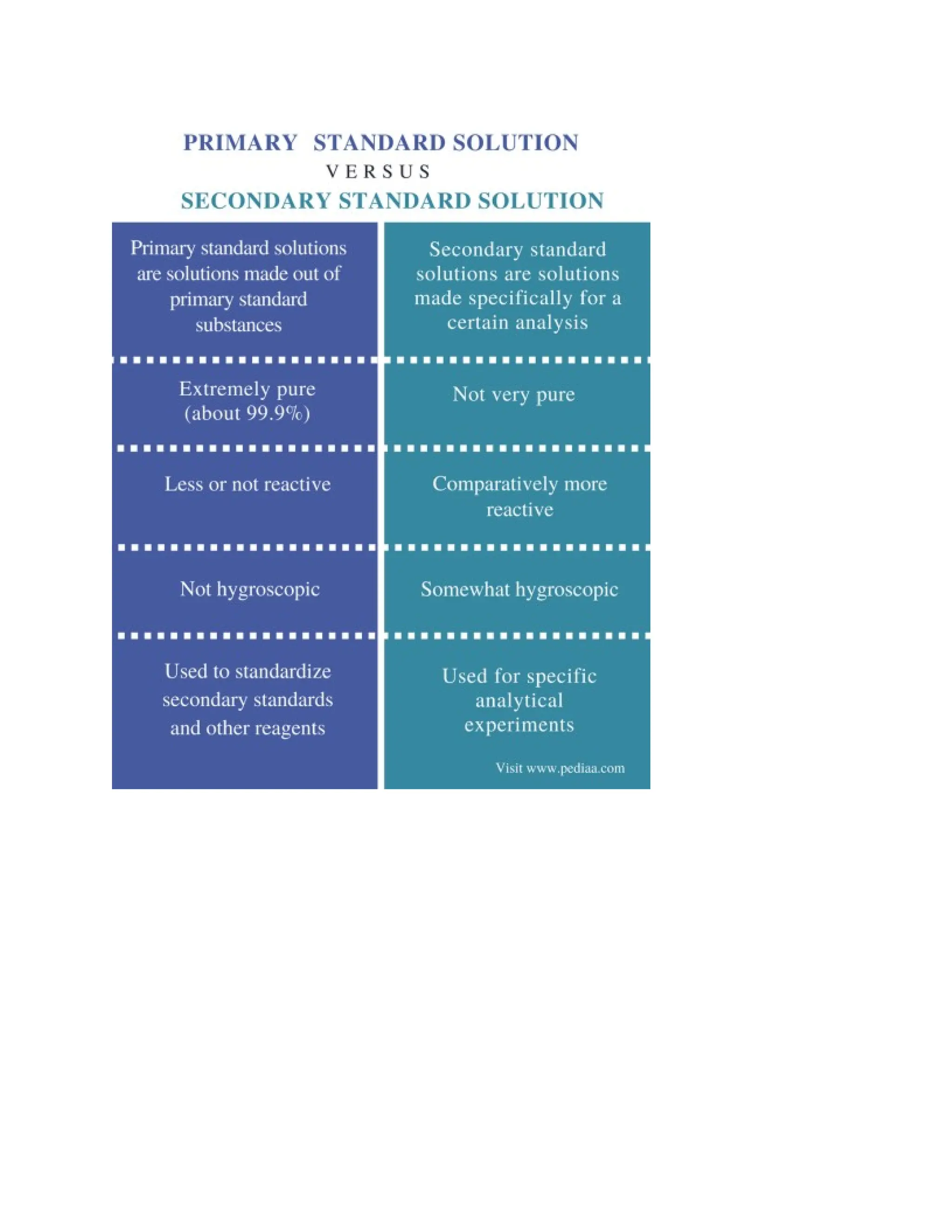
Primary standards are highly accurate, stable, pure substances used as reference points for calibration and measurement, with examples including potassium dichromate and sodium carbonate. Secondary standards, on the other hand, are calibrated instruments or materials that extend the calibration capabilities of primary standards and are easier to handle. They are essential for verifying accuracy in chemical analyses, particularly in pharmaceutical applications.