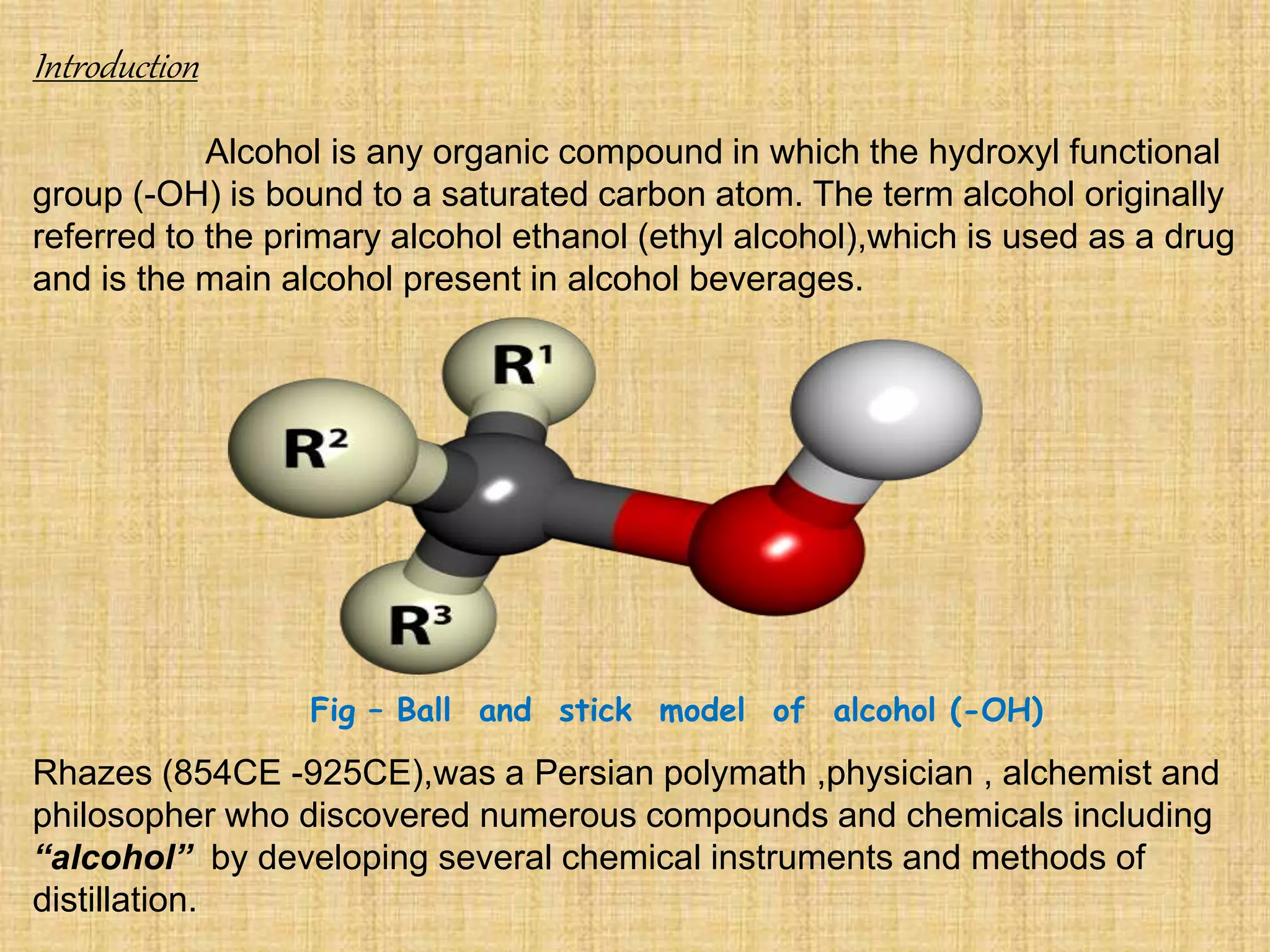
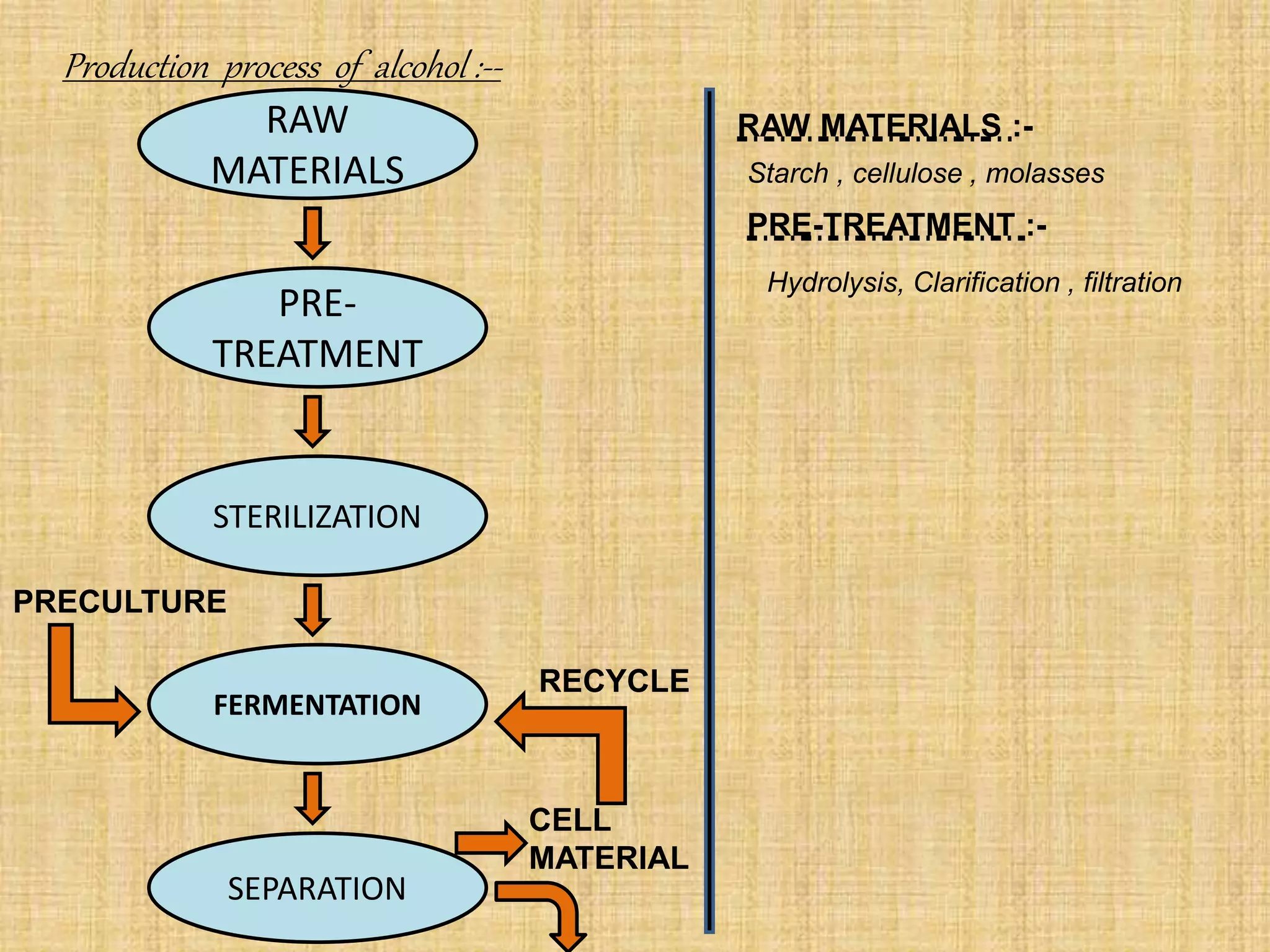
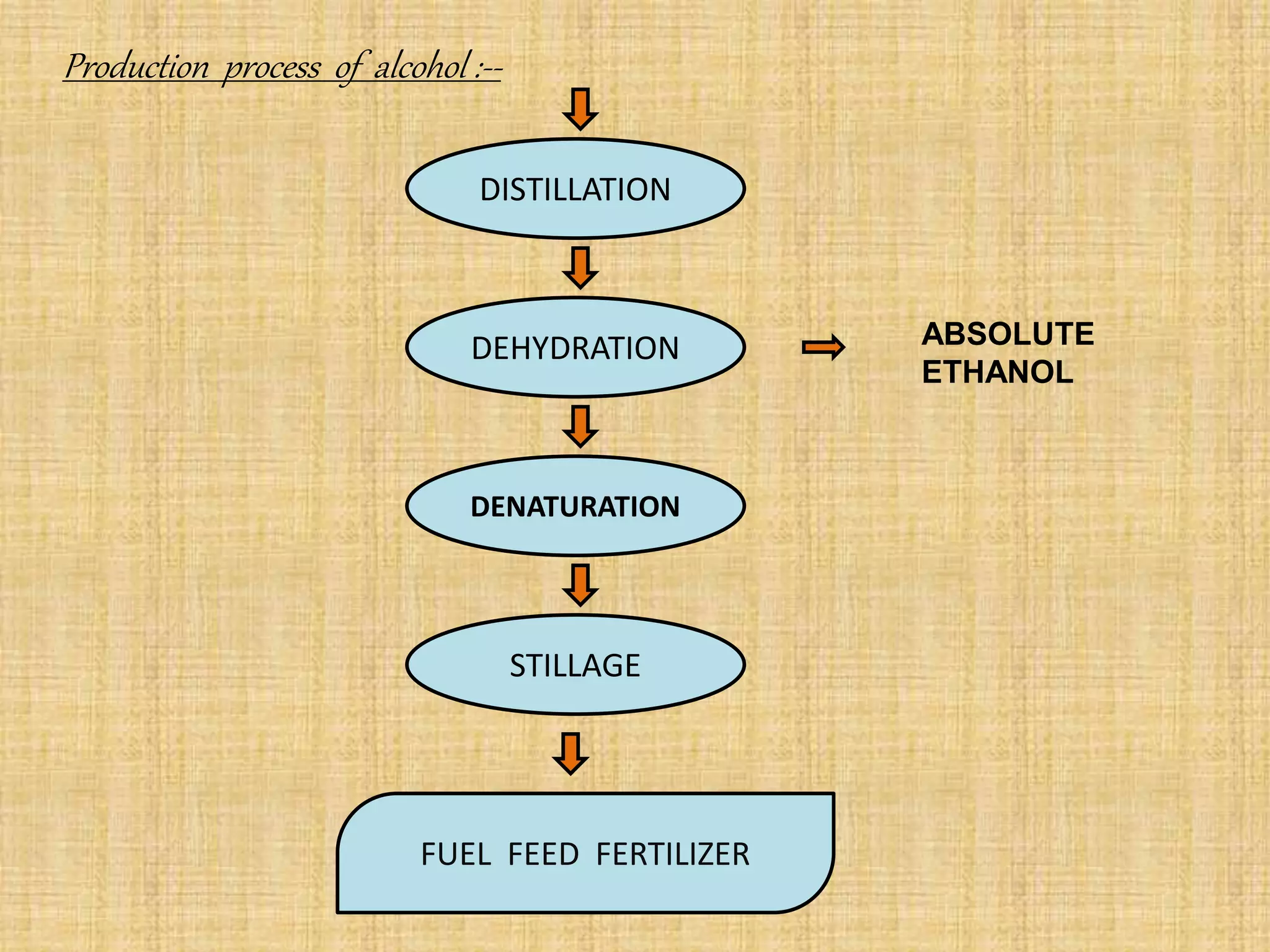
This document summarizes the preparation and production of alcohol. It begins with an introduction to alcohol and its structure. The raw materials and microorganisms used are sugary and starchy materials along with yeast and bacteria. The production process involves pretreating and hydrolyzing the raw materials, fermenting with microorganisms, distilling the alcohol produced, and optionally denaturing it. Finally, the major applications of alcohol are discussed, including use in alcoholic beverages, antifreeze, medicine, fuel, and as a solvent.