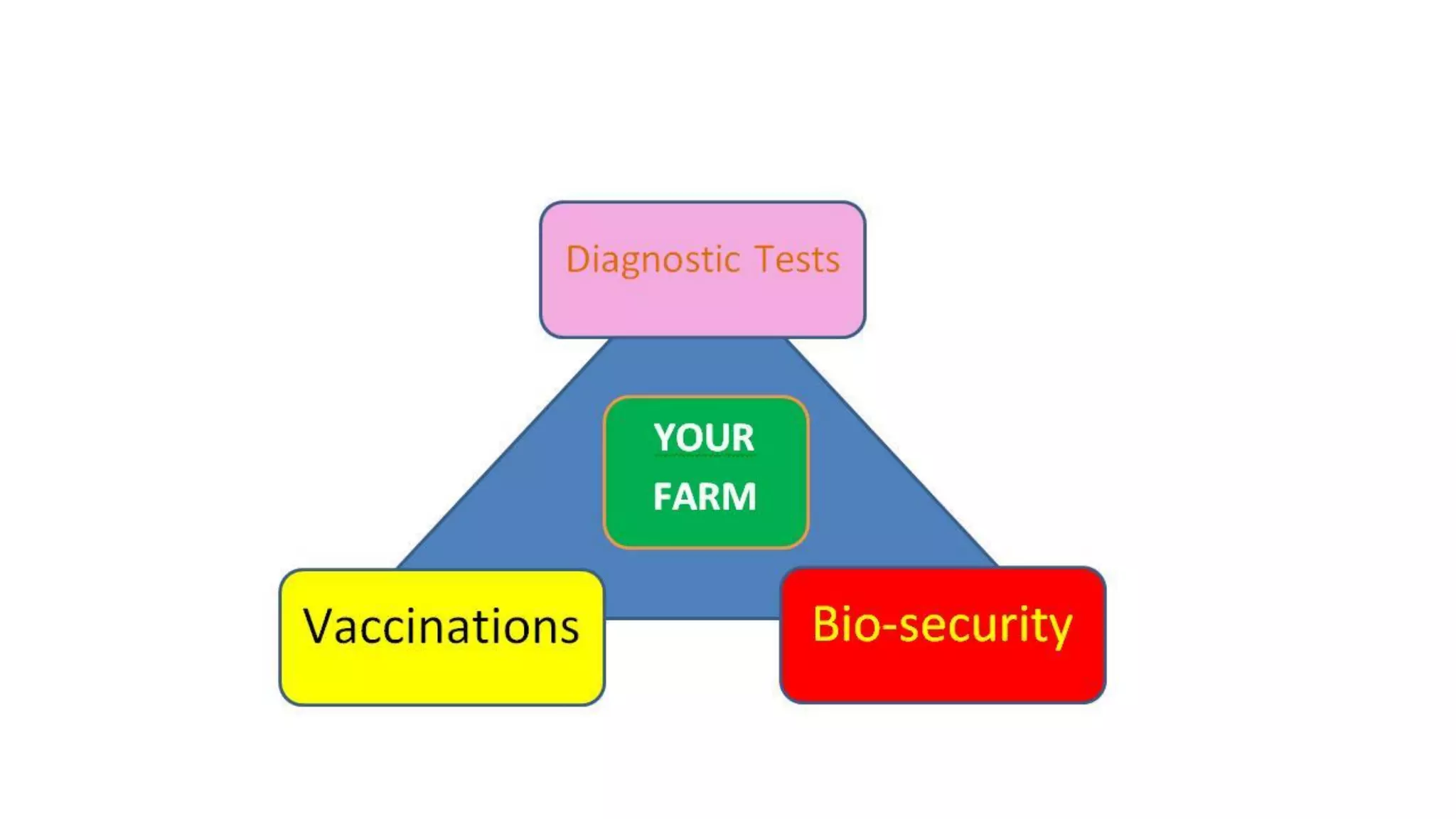
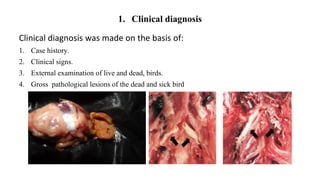
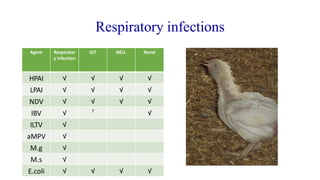
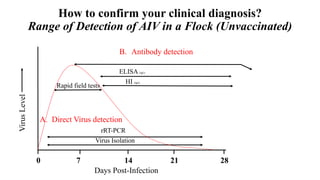
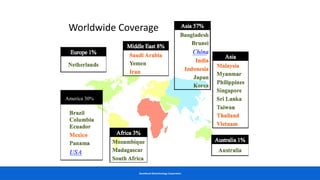
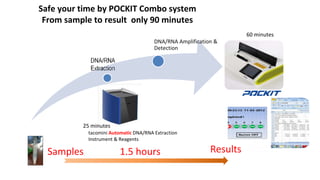
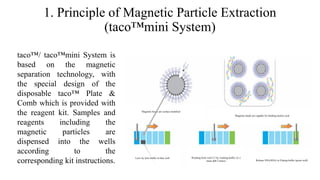
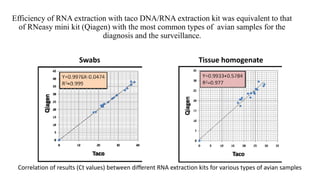
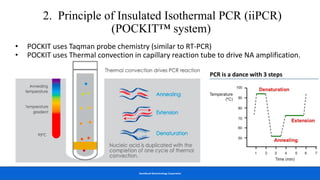
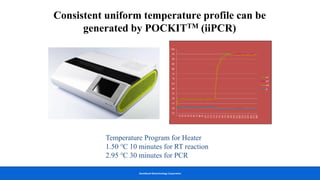
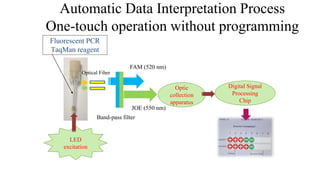
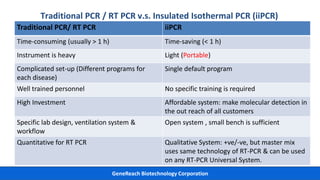
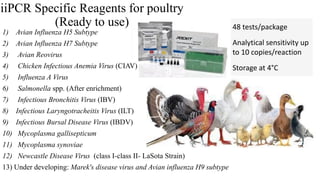
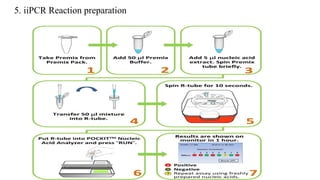
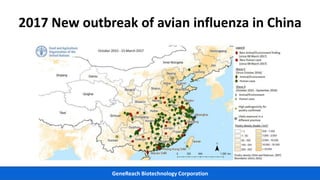
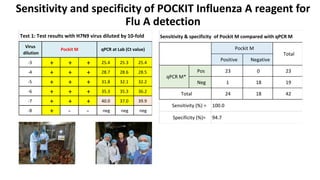
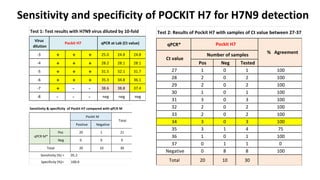
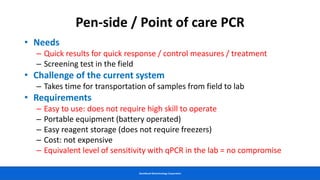
This document discusses practical approaches for diagnosing viral diseases in poultry, including clinical diagnosis, rapid field diagnostic tests, serological diagnosis, molecular diagnosis, and isolation/characterization. Clinical diagnosis is based on case history, clinical signs, examination of live/dead birds, and gross lesions. Rapid field tests can detect viruses but require high viral titers. Serological tests detect antibodies but have delays. Molecular diagnosis using PCR technologies can sensitively and specifically detect pathogens. The document emphasizes that clinical signs alone are not confirmatory and that multiple diagnostic approaches should be used to accurately diagnose poultry viral diseases.