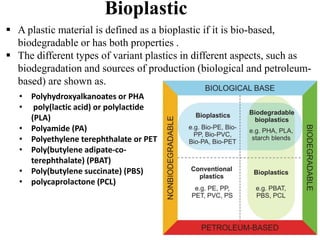
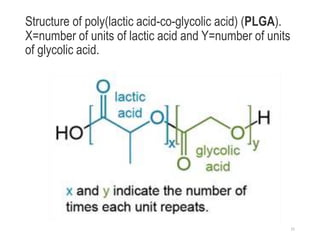
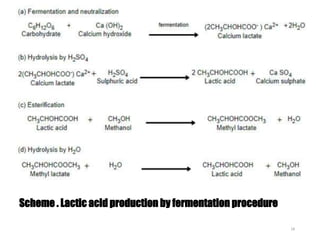
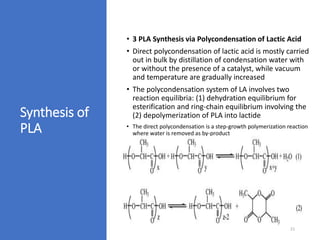
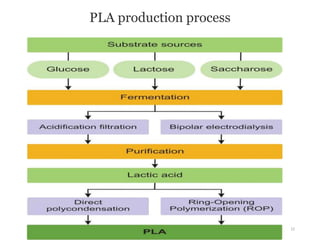
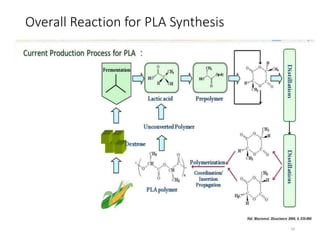
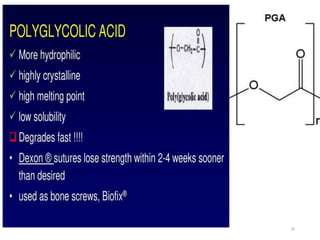
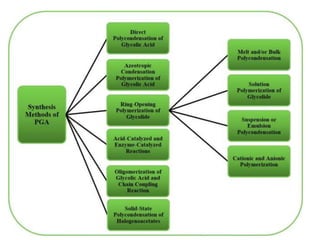
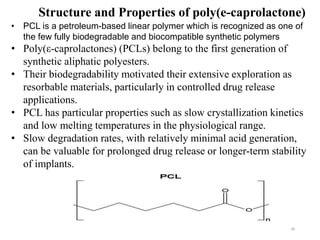
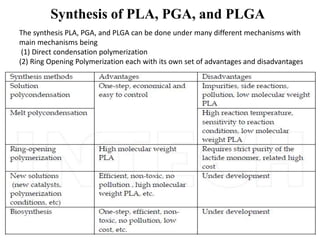
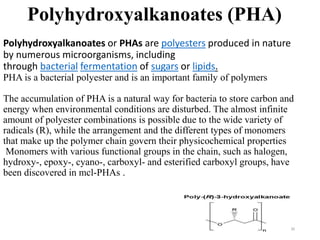
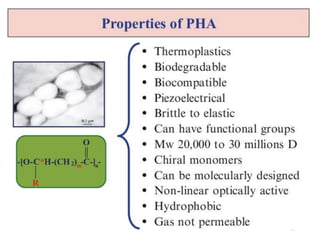
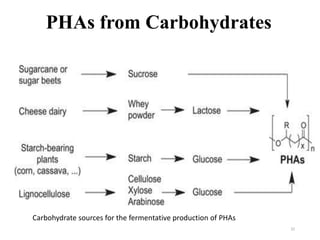
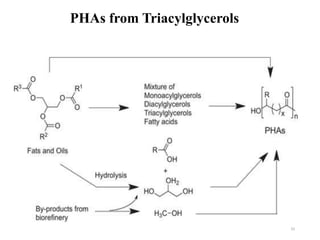
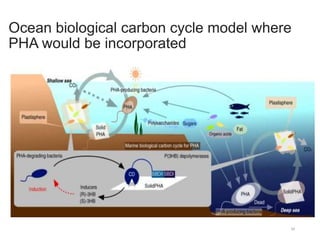
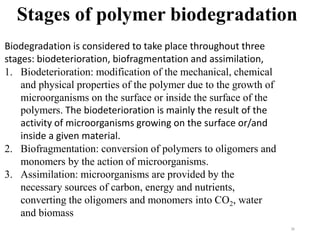
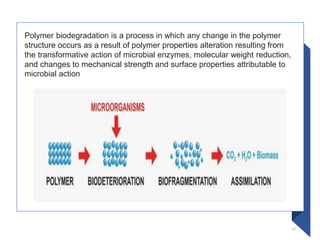
The document discusses various biodegradable plastics including polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL), and polyhydroxyalkanoates (PHAs). It describes the production processes of PLA, PGA, and their copolymer PLGA from renewable resources through fermentation and polymerization. The document also outlines the properties, structures, and key applications of these biodegradable plastics in packaging, medical devices, and tissue engineering.