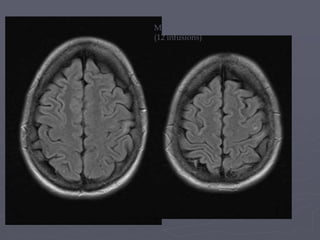
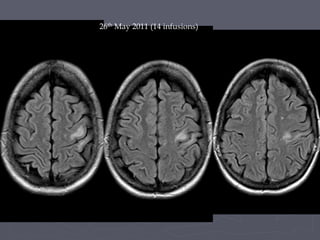
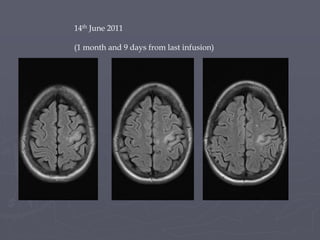
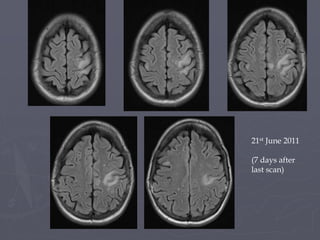
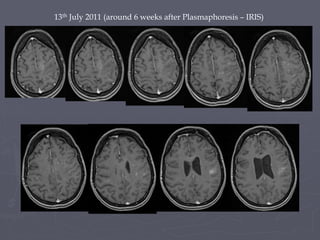
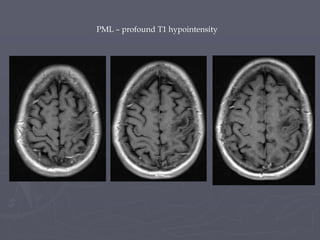
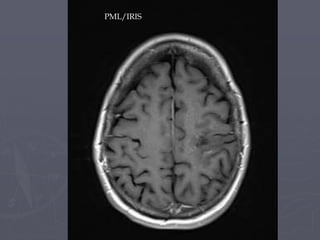
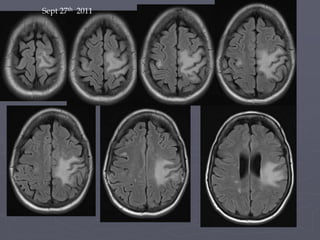
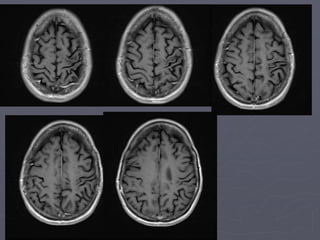
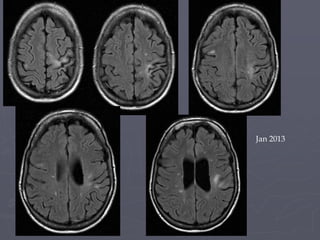
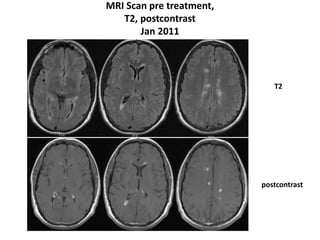
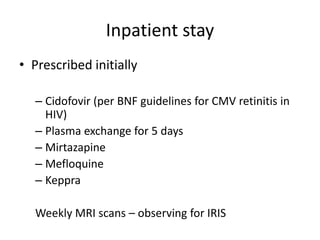
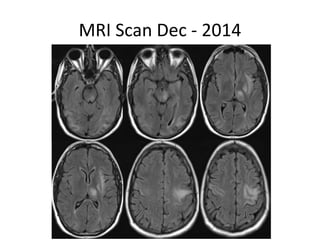
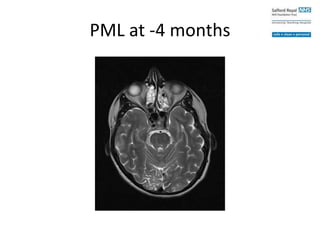
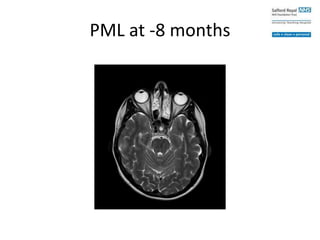
This case study describes a patient with relapsing-remitting MS who was treated with natalizumab (Tysabri) between 2010-2011. In March 2011, she had an abnormal brain lesion detected on a surveillance MRI. Over the next few months her condition progressively declined, with developing weakness and sensory symptoms. Further MRIs and lumbar punctures were consistent with a diagnosis of progressive multifocal leukoencephalopathy (PML) induced by her treatment with natalizumab. She underwent plasma exchange treatment but continued to deteriorate clinically.
















![Risk management
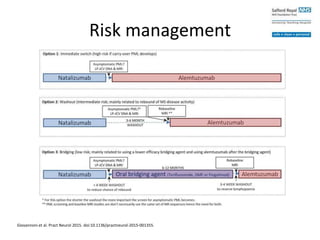
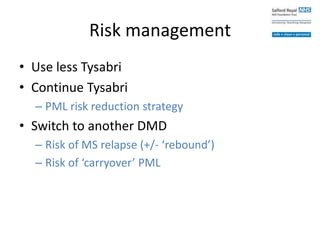
• Switch to another DMD
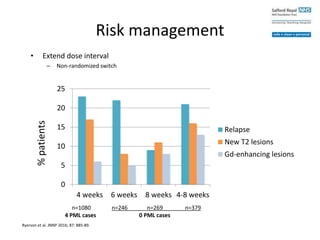
– Non-randomized
– Clinically stable
– Stop/switch due to PML risk
Continuing Tysabri (n=196)
Stop/switch Tysabri (n=122)
[No DMD = 12, Gilenya = 55,
Copaxone = 36, IFNβ = 12,
Mitox = 2, AZA = 2, CYC = 2,
Rituximab = 1]
Prosperini et al. MSJ 2015; 21 (13) 1713-22.](https://image.slidesharecdn.com/managementofpmls-161123163428/85/PML-patient-case-study-Sharon-Letissier-42-320.jpg)