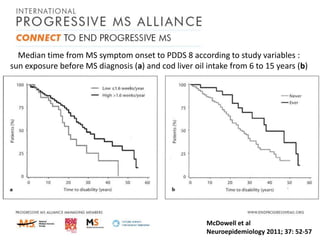
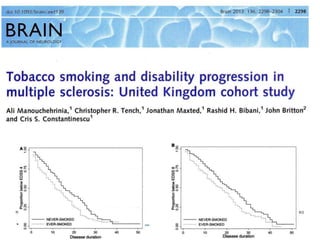
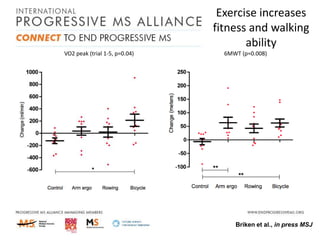
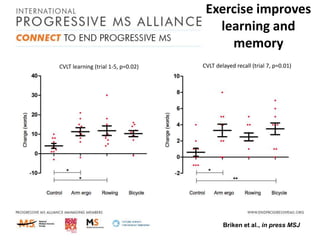
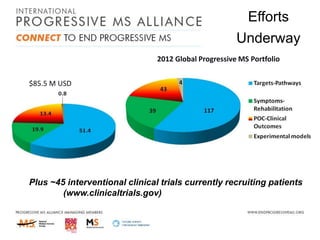
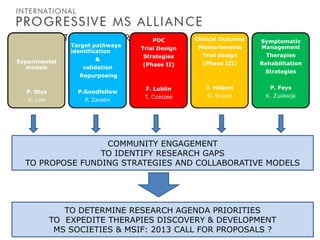
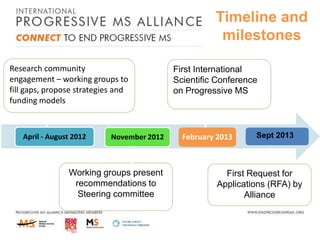
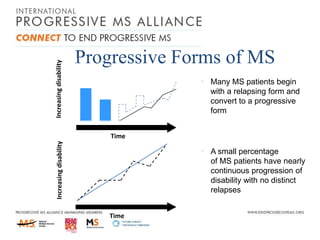
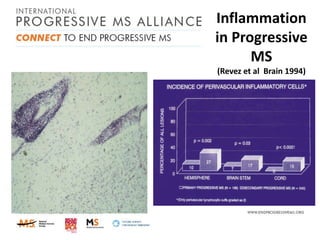
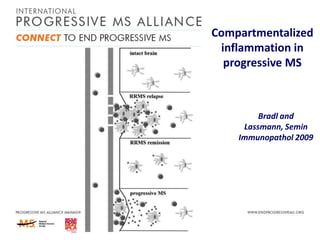
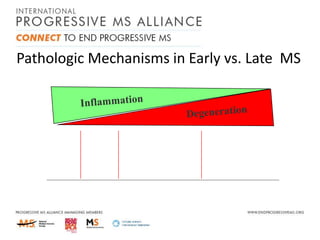
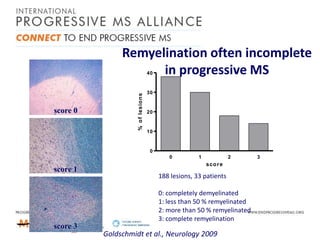
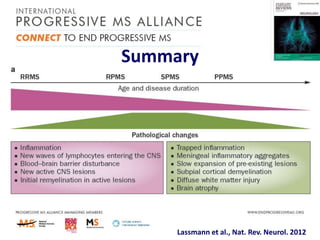
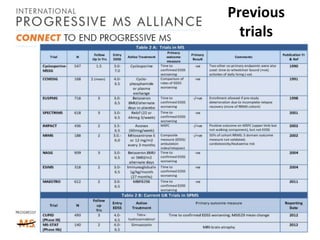
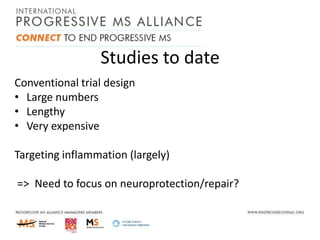
Progressive MS is an unmet clinical need with limited treatment options. This document discusses defining progressive MS, exploring disease mechanisms and interventions, current clinical trials, and the need for an international initiative. It summarizes research showing inflammation, demyelination, gray matter involvement, and axonal loss contribute to progression. Clinical trials have targeted inflammation with limited success. Future trials aim to test neuroprotective agents, lifestyle factors, and remyelination/rehabilitation approaches. An international collaborative is needed to expedite therapies through target identification, preclinical models, clinical outcome measures, trial design, and engaging the research community. The goal is to accelerate development of effective treatments for disease modification and symptom management in progressive MS.











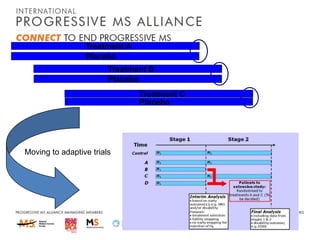









![MS-STOP>>MS-SMART
4 arms [1 placebo + 3 active]
Multiplex Phase IIb trial
– 4*110=440
– allowing for drop-outs [10%+10%]
– Primary outcome=SIENA PBVC
– Gives 90% power for 35% treatment effect](https://image.slidesharecdn.com/alanthompson-131219063720-phpapp02/85/Targeting-Progession-The-Progressive-MS-Alliance-54-320.jpg)