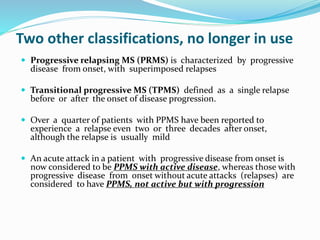
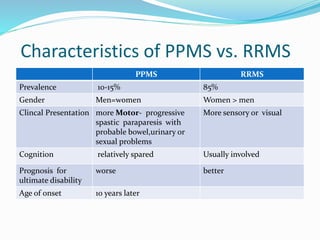
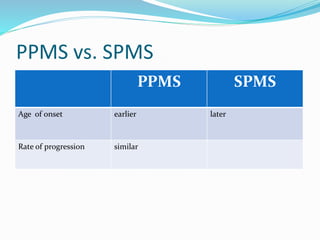
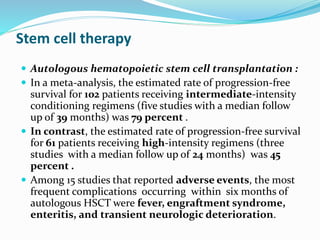
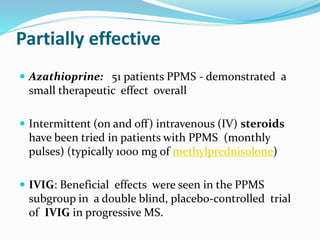
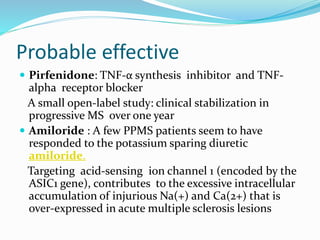
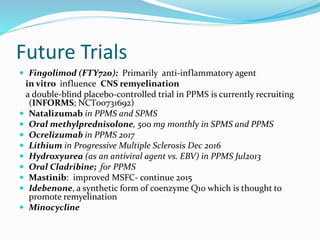
Primary progressive multiple sclerosis (PPMS) is characterized by progressive accumulation of disability from disease onset, with occasional plateaus or improvements. PPMS is diagnosed based on 1 year of progression plus MRI or CSF evidence of disseminated lesions. PPMS affects men and women equally and has an average later age of onset than relapsing-remitting MS. While several treatments have failed in PPMS or been ineffective, glatiramer acetate, rituximab, methotrexate, stem cell therapy, and low-dose naltrexone may be partially effective in subsets of patients. Future trials are exploring therapies such as fingolimod, natalizumab, ocrelizumab, and ib