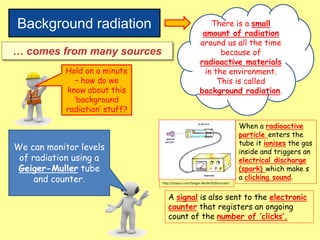
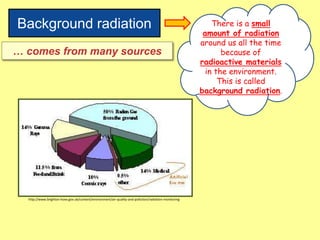
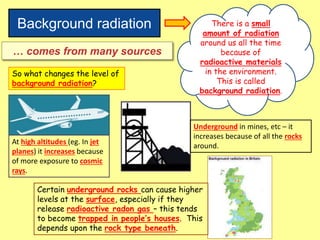
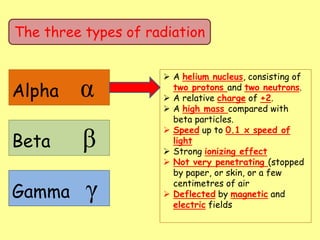
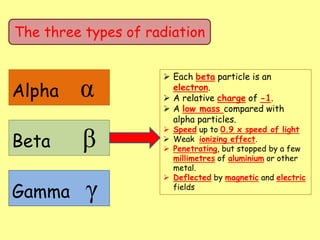
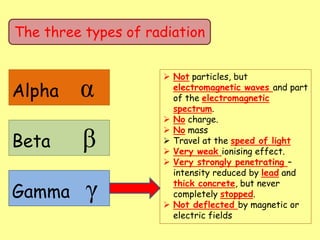
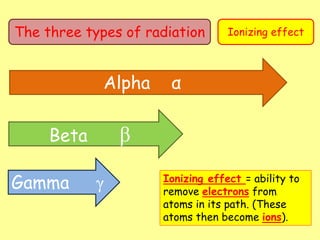
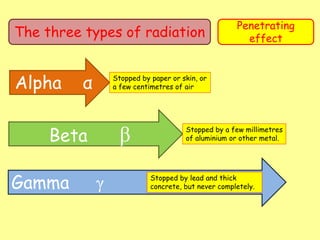
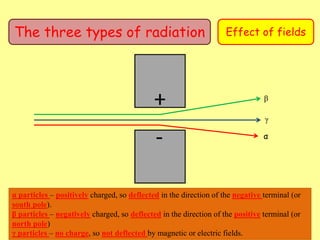
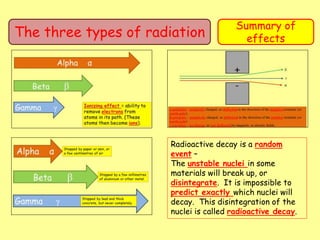
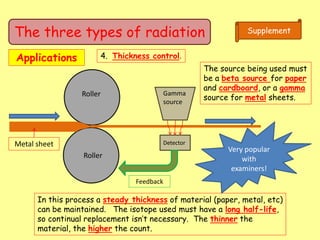
The document discusses background radiation and the three types of radiation: alpha, beta, and gamma. It provides details on their nature, ionizing effects, penetrating abilities, and how they are affected by electric and magnetic fields. Applications of alpha, beta, and gamma radiation are also summarized, including use as medical tracers, industrial tracers, food sterilization, thickness control in manufacturing, and cancer radiotherapy. The document aims to describe the core concepts and properties of different radioactive emissions.