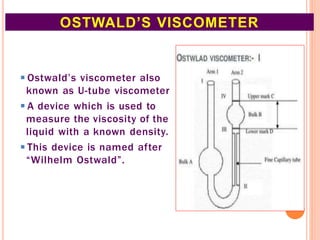
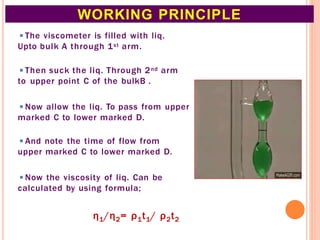
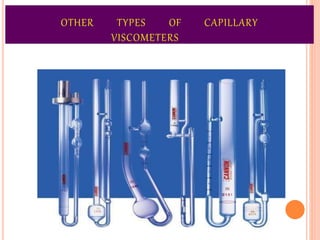
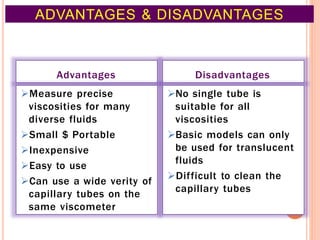
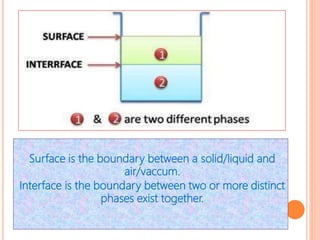
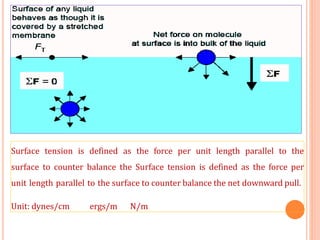
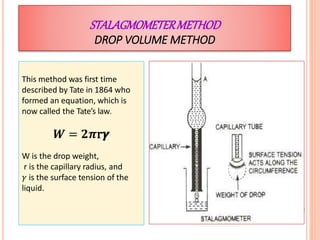
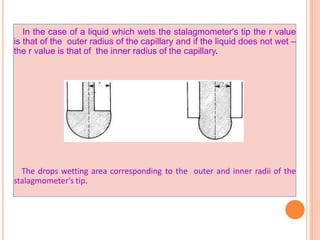
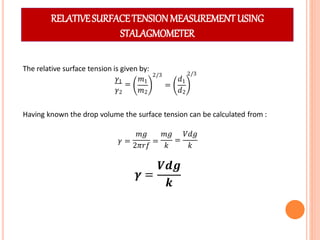
This document discusses viscosity measurement techniques. It describes viscometers like the capillary viscometer and Ostwald's viscometer. The capillary viscometer uses Poiseuille's law to measure the time taken for a fluid to flow through a capillary tube, compared to a standard fluid. Ostwald's viscometer is a U-tube device that measures the time for a liquid to flow between two points at different heights. Surface tension is also examined using the stalagmometer drop volume method based on Tate's law. Precautions for accurate surface tension measurements are provided.