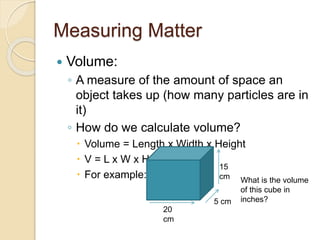
This document defines key properties of matter - mass, volume, density - and how to measure them. It explains that matter is anything that has mass and takes up space, and defines mass vs weight. Volume is measured as length x width x height or via water displacement. Density is the ratio of an object's mass to its volume, showing how tightly packed its particles are. The document provides examples of calculating volume, density, and identifying unknown materials based on their measured density.