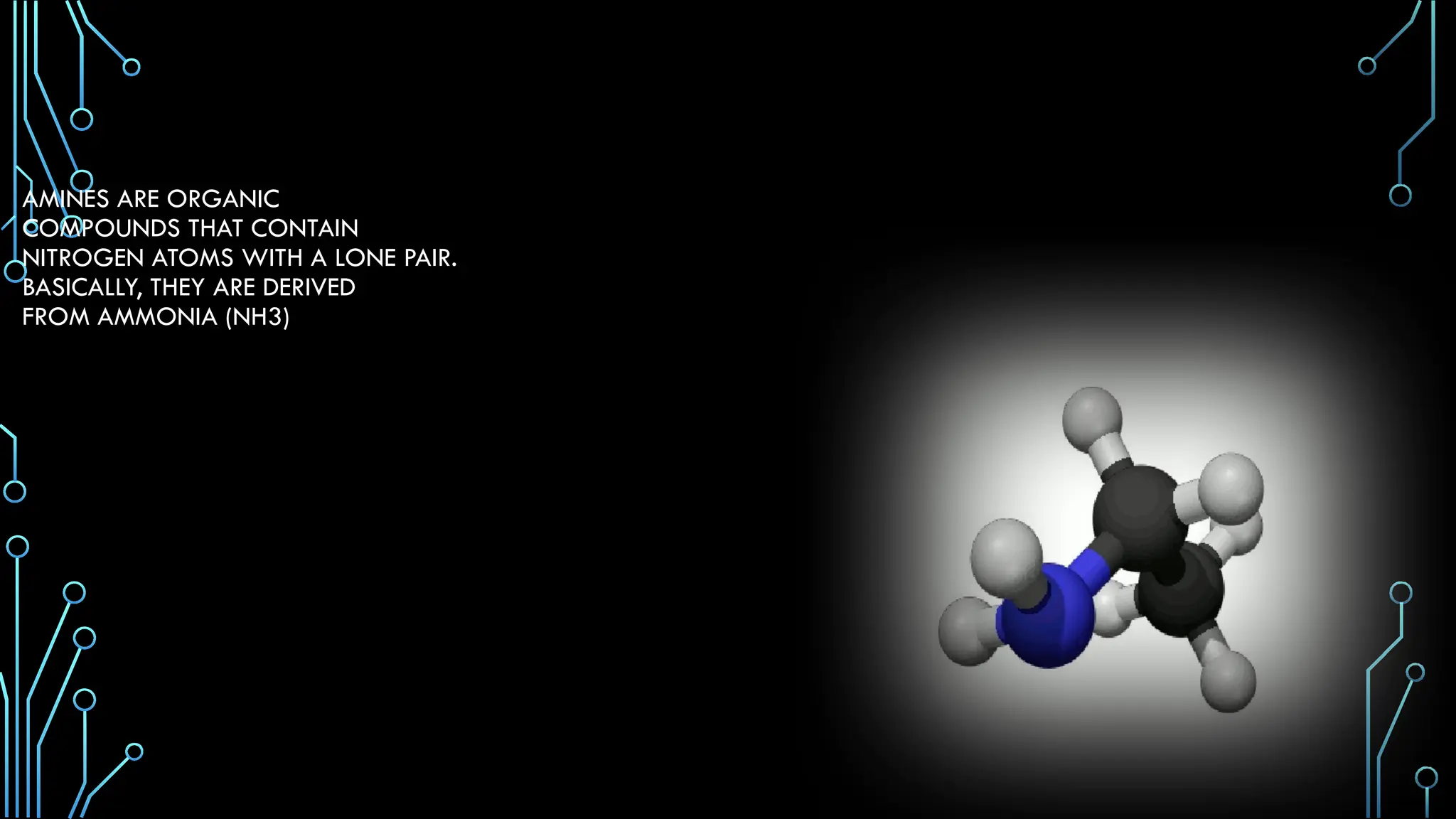
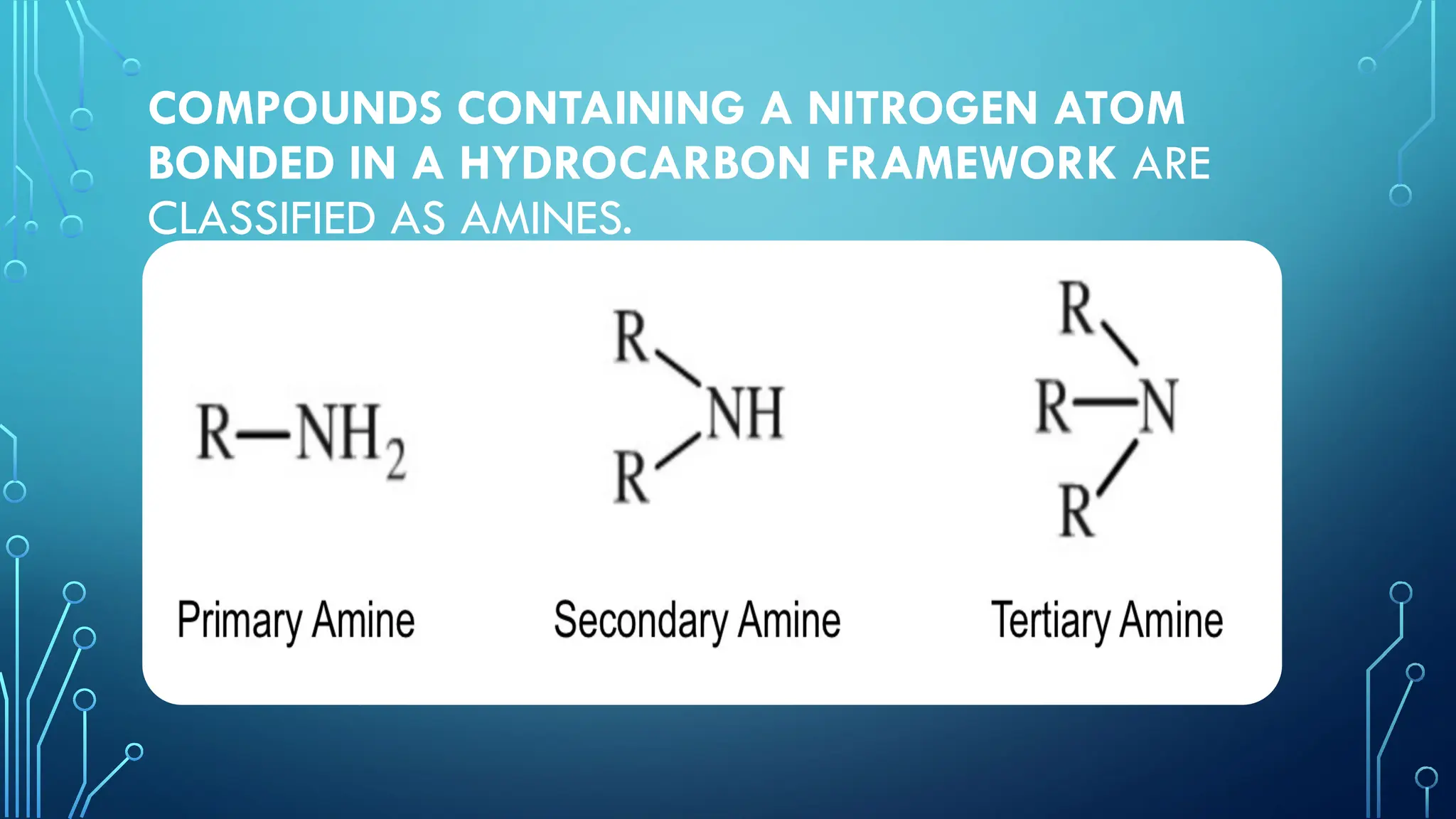
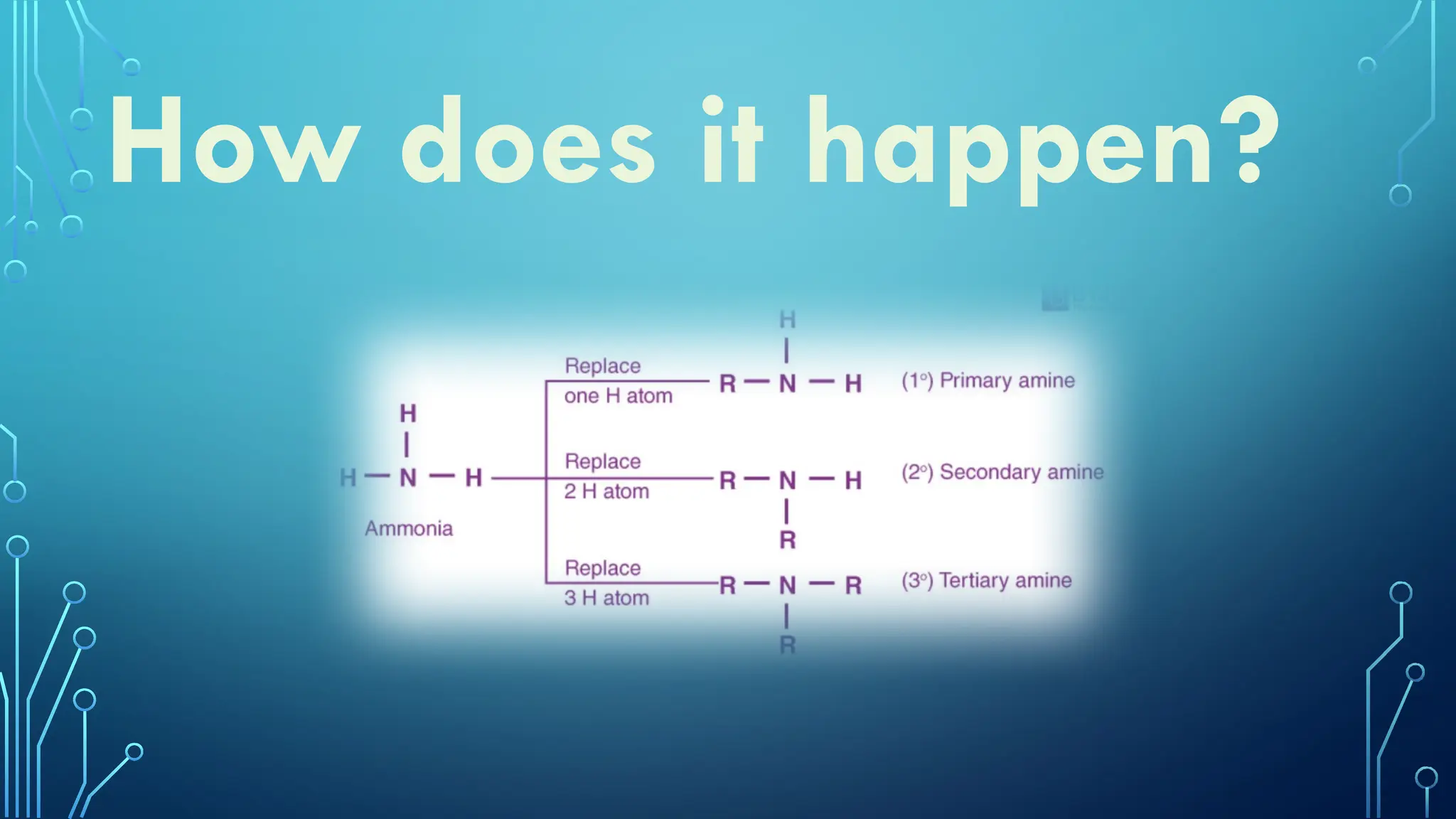
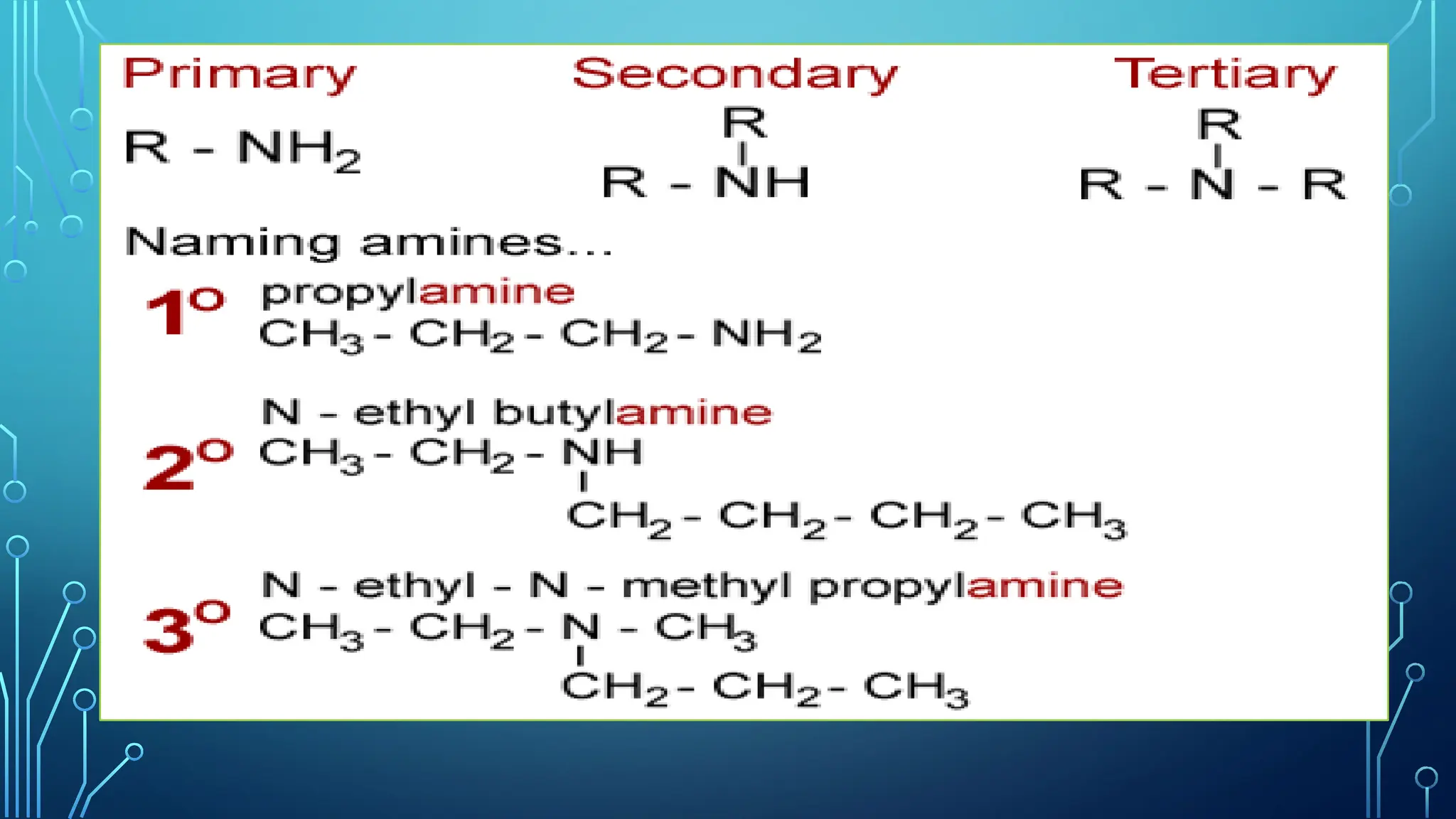
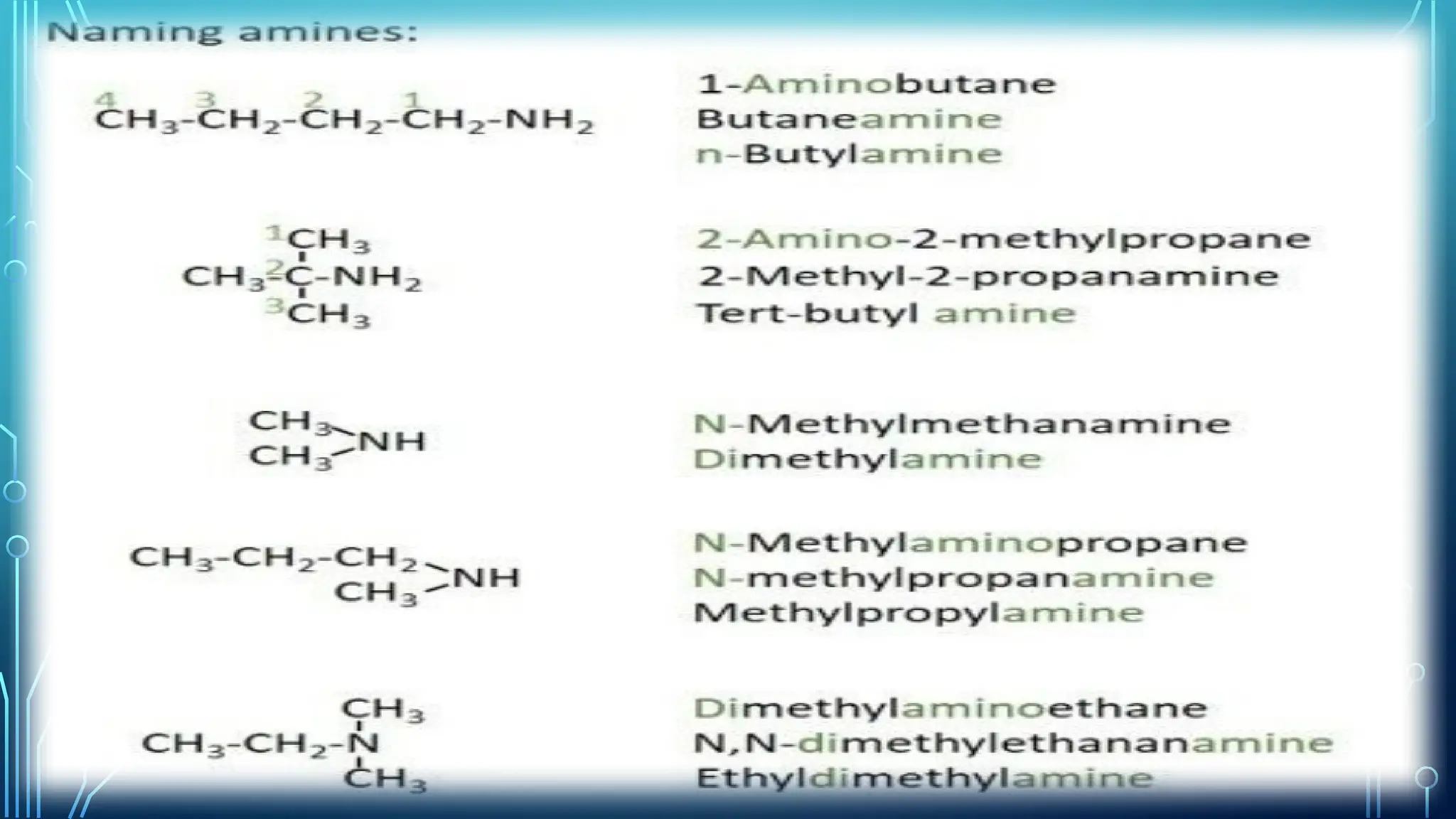
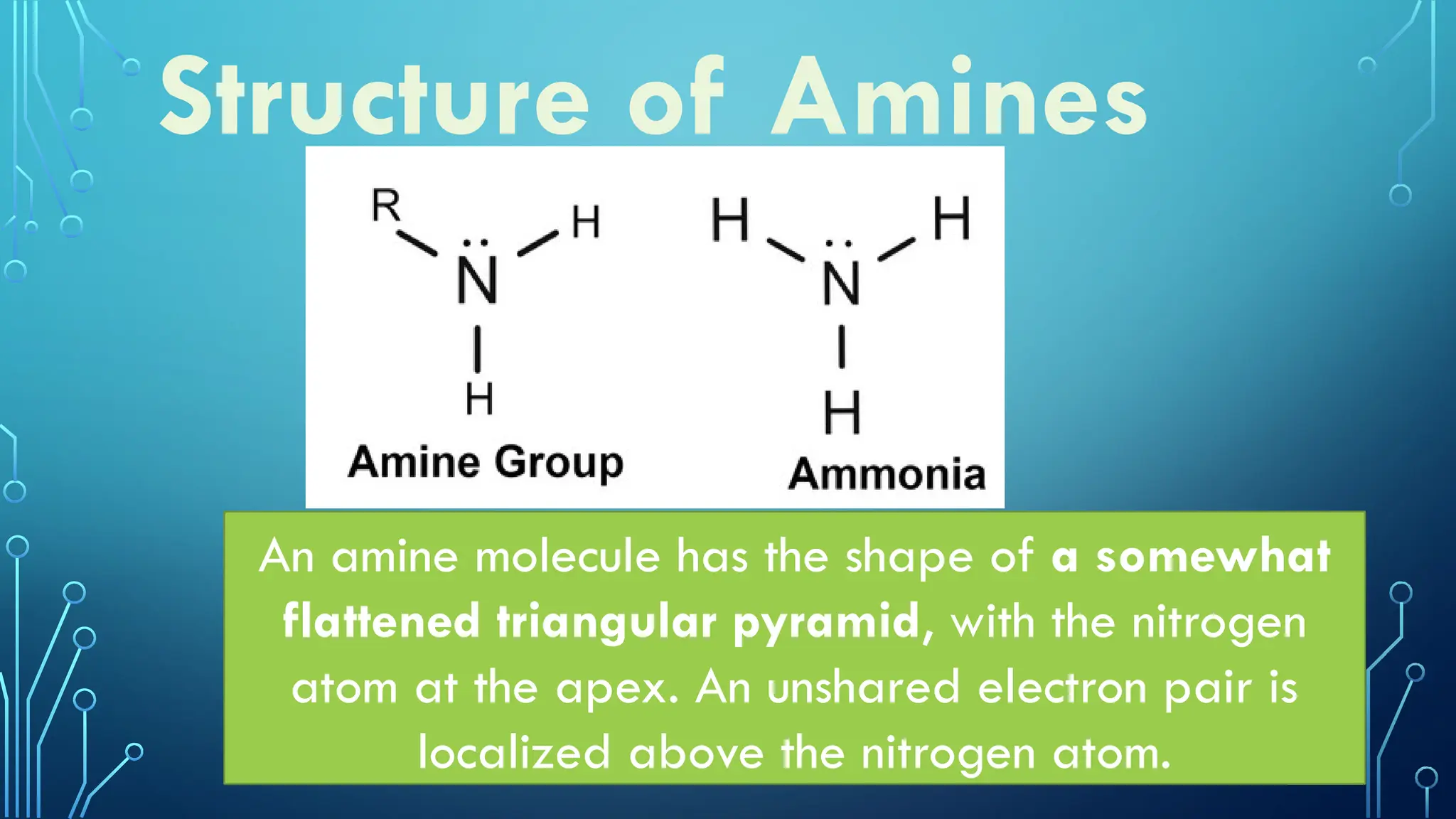
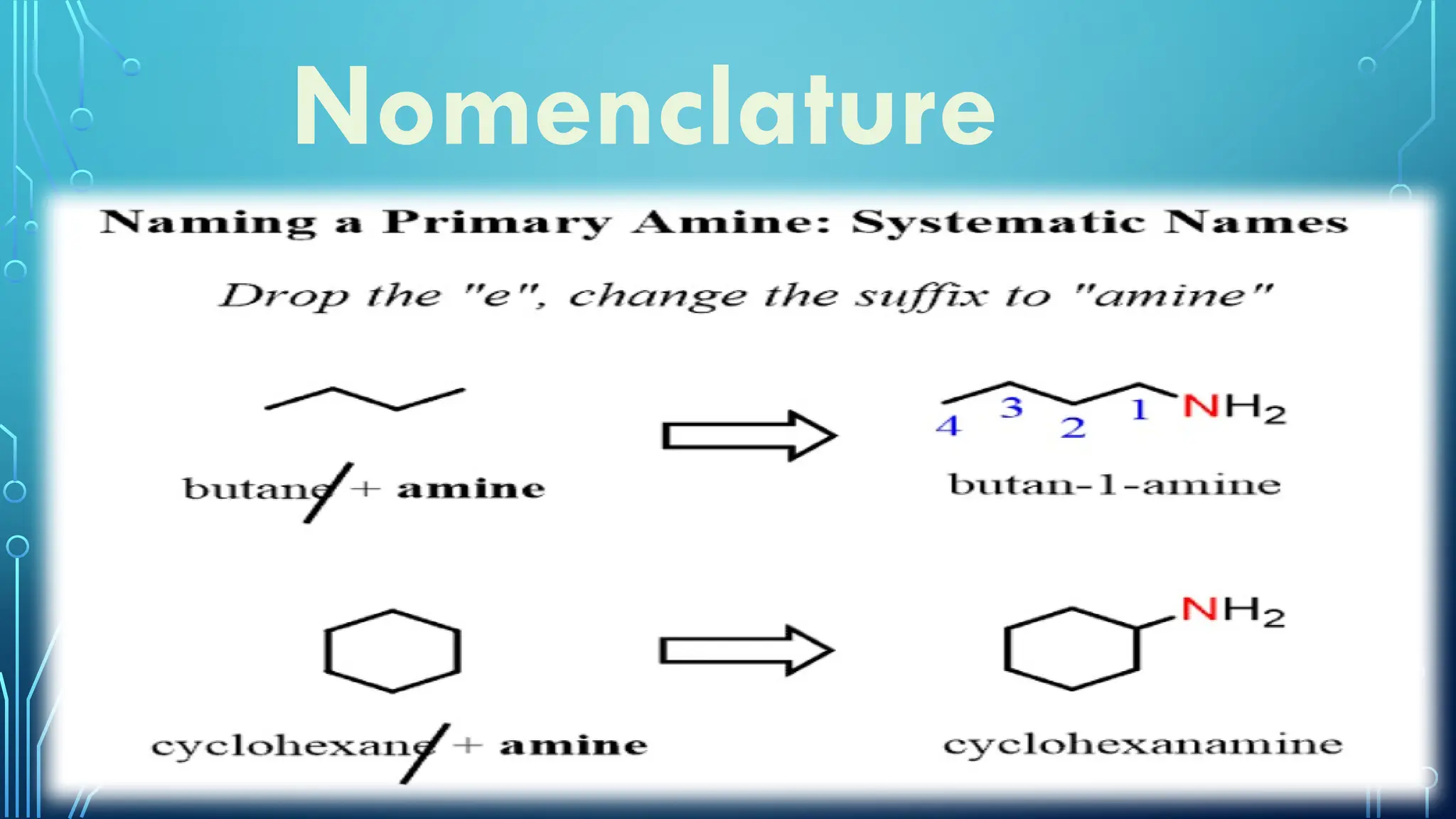
Amines are organic compounds containing nitrogen atoms derived from ammonia, characterized by their unique naming conventions based on substituents. They have a triangular pyramidal structure with an unshared electron pair at the nitrogen apex and are classified as primary, secondary, or cyclic amines based on their structure. Amines play significant roles in nature and synthetic applications, found in proteins, hormones, and various industrial products like dyes and drugs.