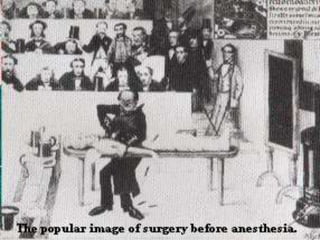
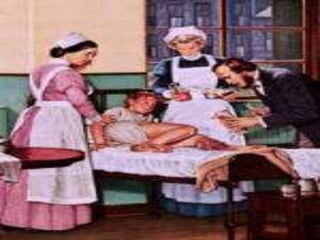
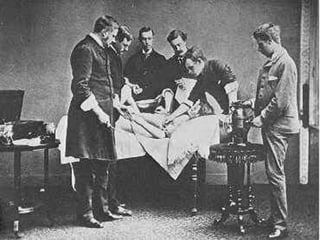
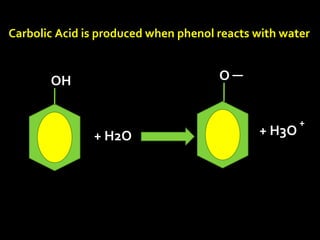
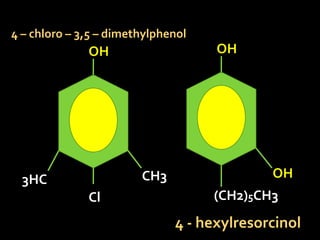
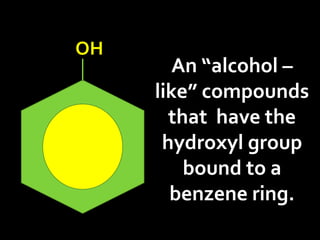
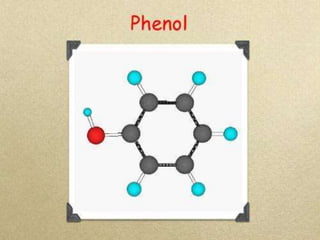
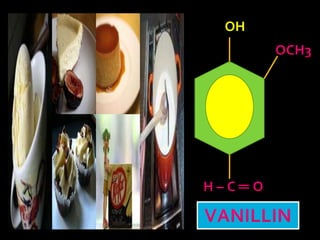
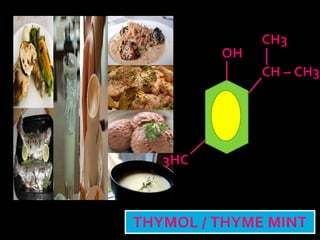
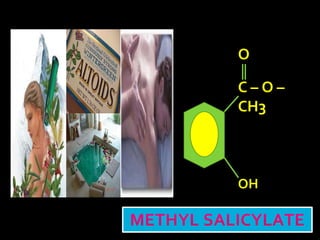
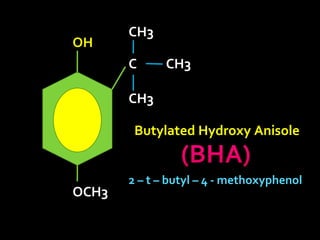
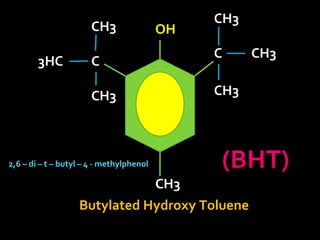
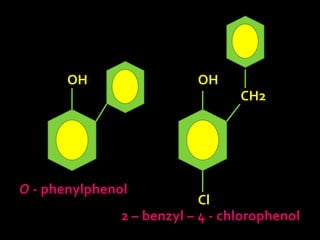
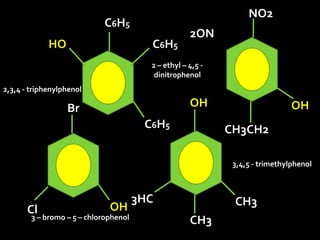
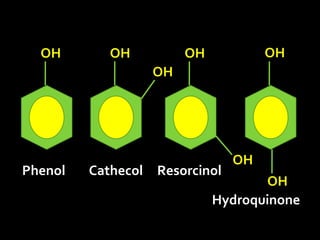
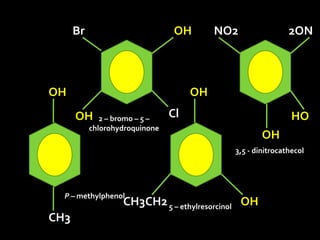
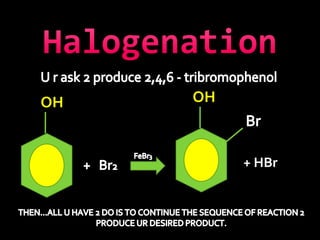
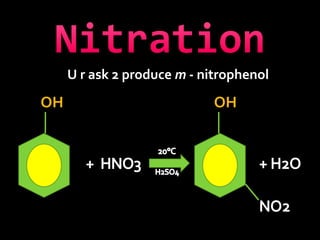
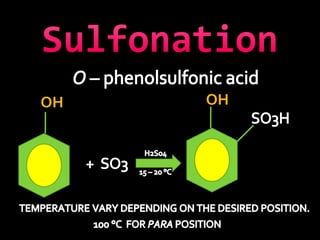
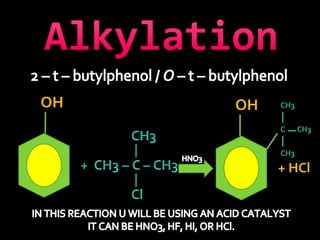
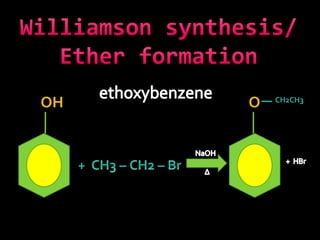
In the 1800s, hospitals were often dangerous places for patients due to poor sanitation practices of doctors. Doctors did not wash their hands or wear protective clothing, and surgery was performed without anesthesia. This led to many postoperative infections that often caused patient deaths. Joseph Lister later discovered that spraying carbolic acid in operating theaters could kill germs and greatly reduce infection rates after surgery, saving many lives. He is now considered the father of antiseptic surgery.