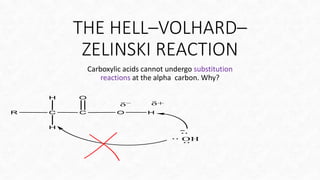
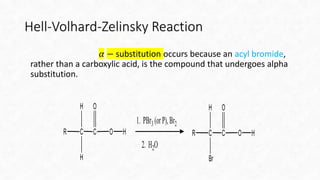
The Hell-Volhard-Zelinsky (HVZ) reaction allows for the bromination of the alpha carbon of a carboxylic acid when treated with PBr3 and Br2, by first converting the acid to an acyl bromide. The reaction requires harsh conditions, including high temperatures and extended times, to favor the desired substitution. The process involves the formation of an enol that rapidly undergoes bromination before hydrolysis yields an alpha-brominated carboxylic acid.