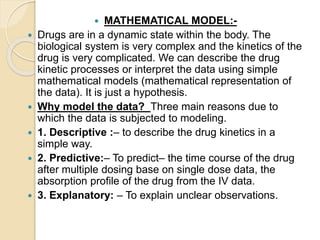
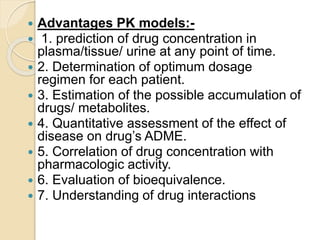
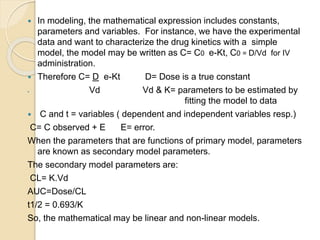
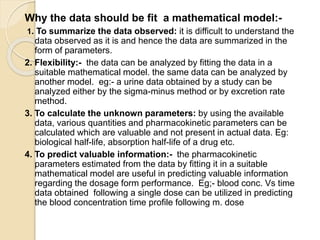
This document discusses pharmacokinetics and mathematical modeling. It defines pharmacokinetics as explaining drug movement in the body using mathematical models. Common models include compartmental models, which divide the body into hypothetical compartments based on drug distribution and movement. The document discusses one-compartment and two-compartment models and their use in estimating parameters like drug concentration over time. It also covers qualities of effective mathematical models and how they are used to analyze pharmacokinetic data.