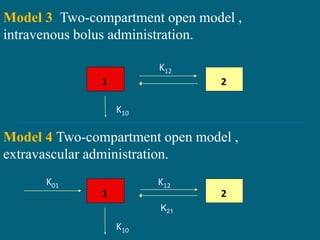
The document presents an overview of pharmacokinetic modeling techniques. It discusses compartment models and non-compartmental analyses that are commonly used to analyze pharmacokinetic data and predict drug absorption, distribution, metabolism and excretion in the body. Compartment models represent the body as a series of hypothetical compartments that drugs move between according to first-order kinetics. Physiologic models group tissues into compartments based on perfusion properties. Non-compartmental analysis uses statistical moments to describe drug disposition without assuming a compartment model.