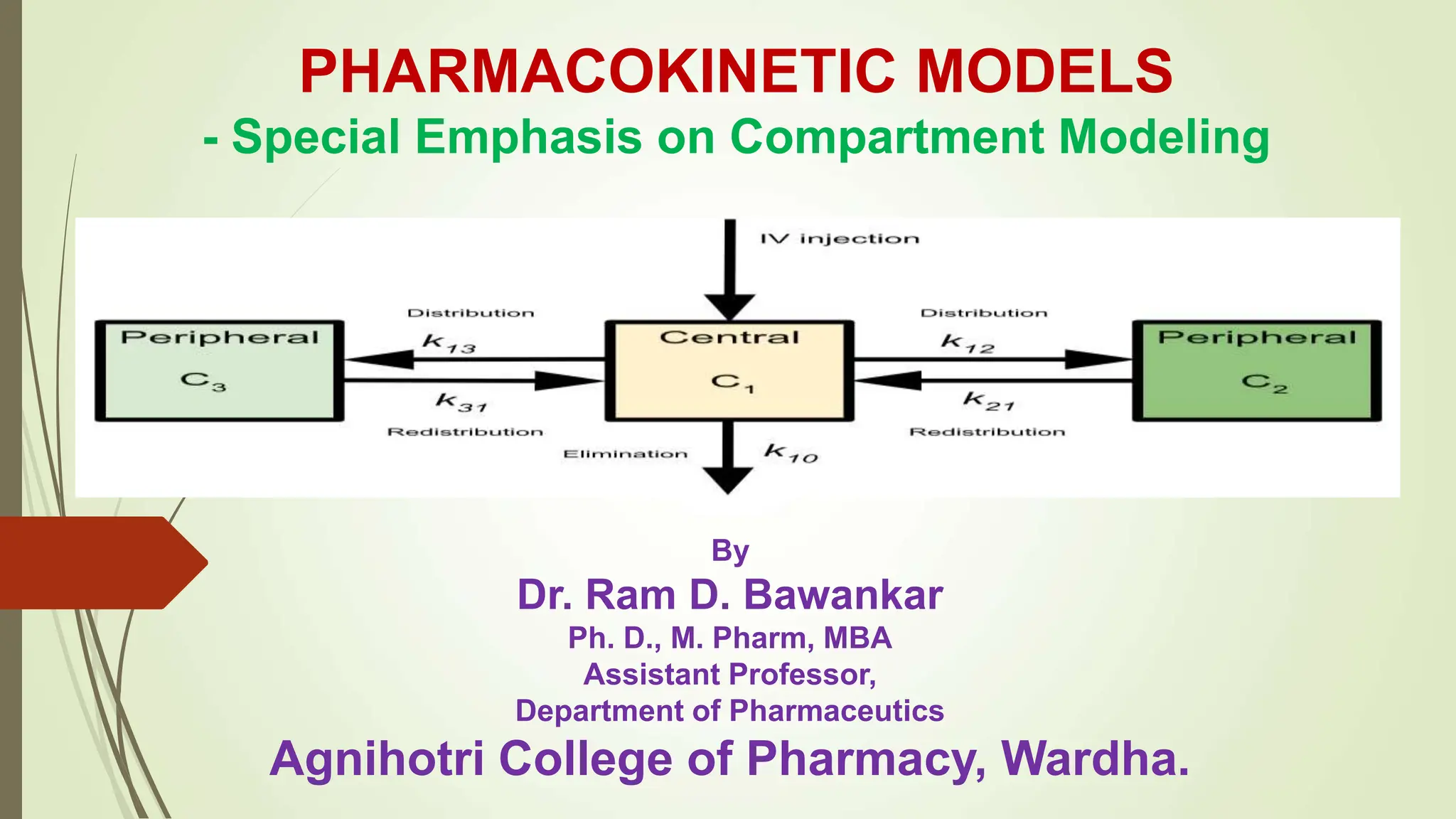
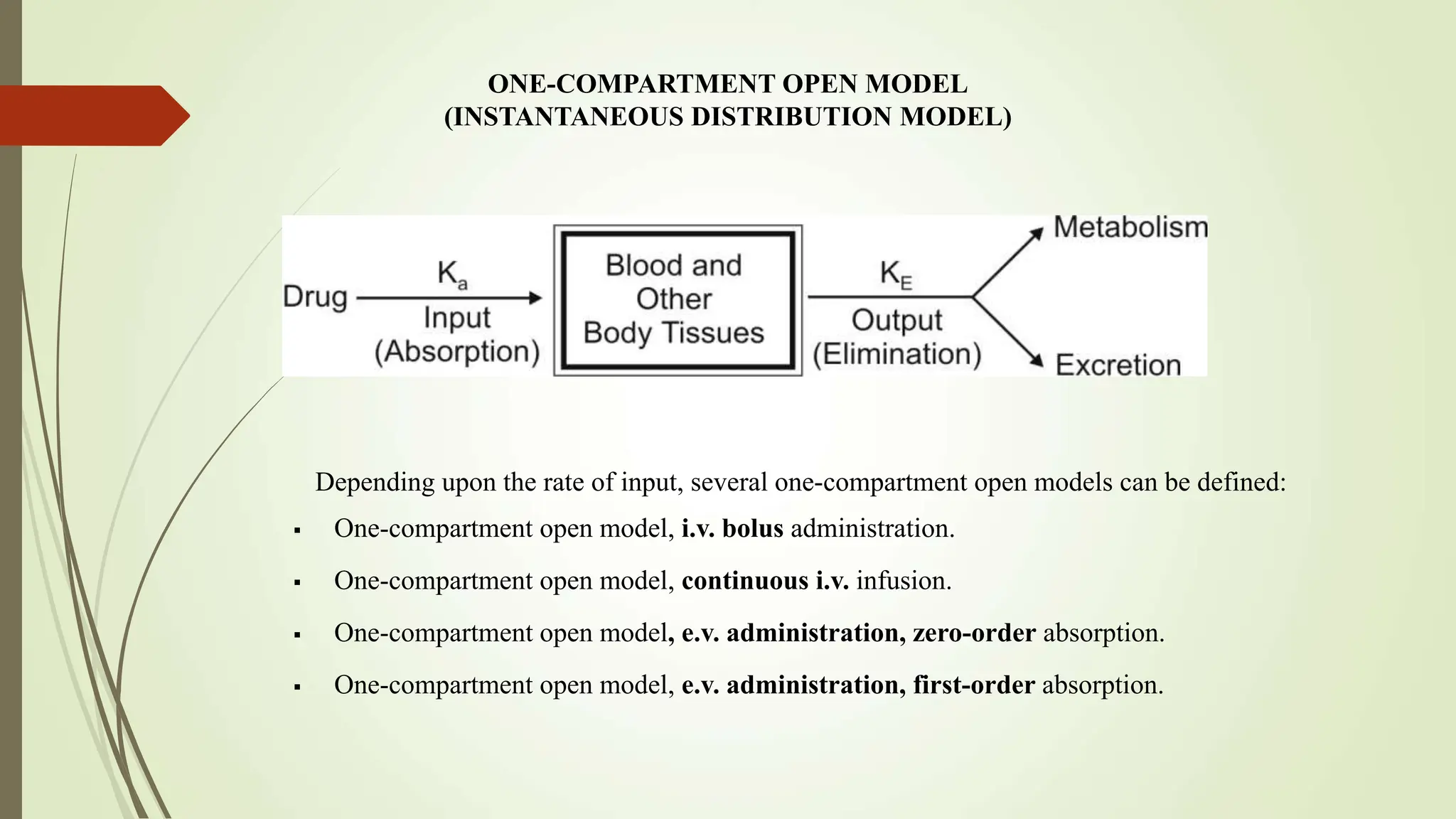
Pharmacokinetic models, including compartment models, are used to quantitatively study how drugs are absorbed, distributed, metabolized and eliminated by the body. Compartmental modeling divides the body into compartments and uses rate constants to describe drug movement between compartments. A one-compartment open model is described which uses first-order kinetics to model drug elimination from a single compartment after intravenous or extravascular administration. Key pharmacokinetic parameters like elimination rate constant, half-life and clearance are defined for this model. Intravenous bolus, intravenous infusion and extravascular administration are discussed in the context of the one-compartment open model.