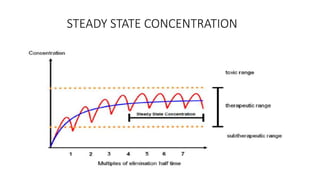
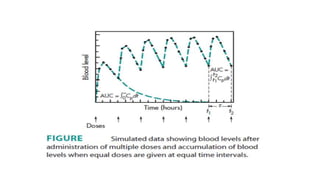
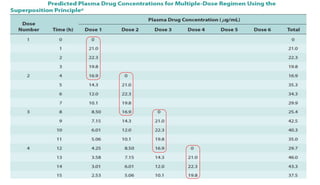
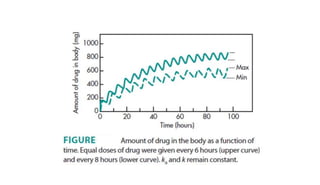
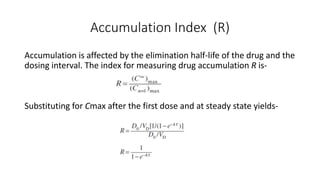
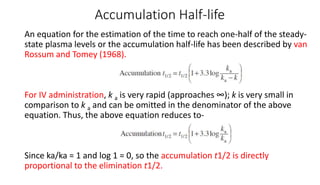
The document discusses the importance of multiple dosage regimens in maintaining plasma drug levels within therapeutic ranges, especially for drugs with short durations of action. It outlines key parameters like dose size and frequency, the concept of steady-state drug concentration, and factors affecting drug accumulation. Additionally, it covers the rationale for using loading doses to achieve target concentrations rapidly, and provides examples of drugs typically administered using these regimens.