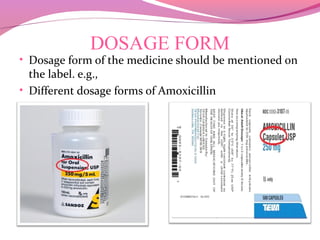
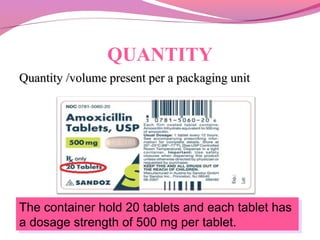
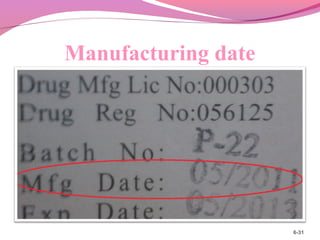
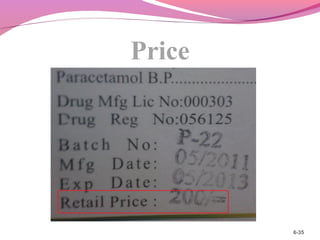
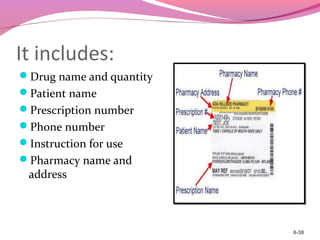
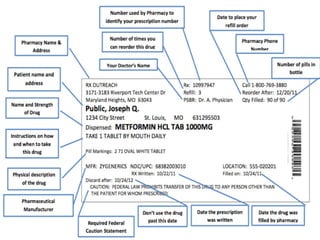
This document discusses the different types of labels required for pharmaceutical products. It describes manufacturer labels, which contain drug information supplied by the manufacturer for medical professionals. Manufacturer labels must include the name, strength, dosage form, quantity, instructions, precautions, warnings, registration number, batch number, manufacturing and expiry dates, and manufacturer information. Dispensing labels contain additional information for patients, such as the patient's name, prescription number, directions for use, and pharmacy details. Proper labeling helps ensure drugs are used safely and effectively.