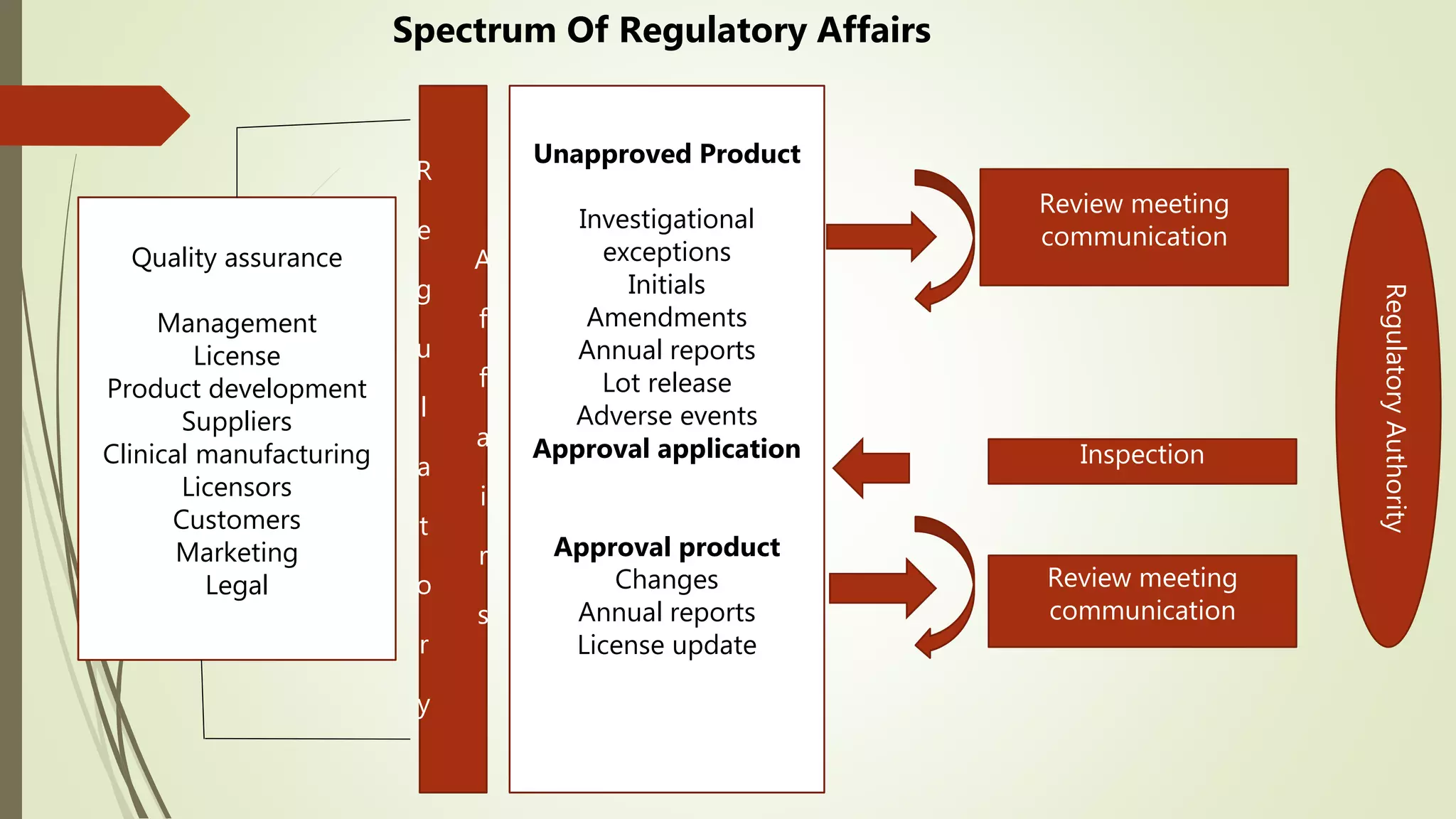
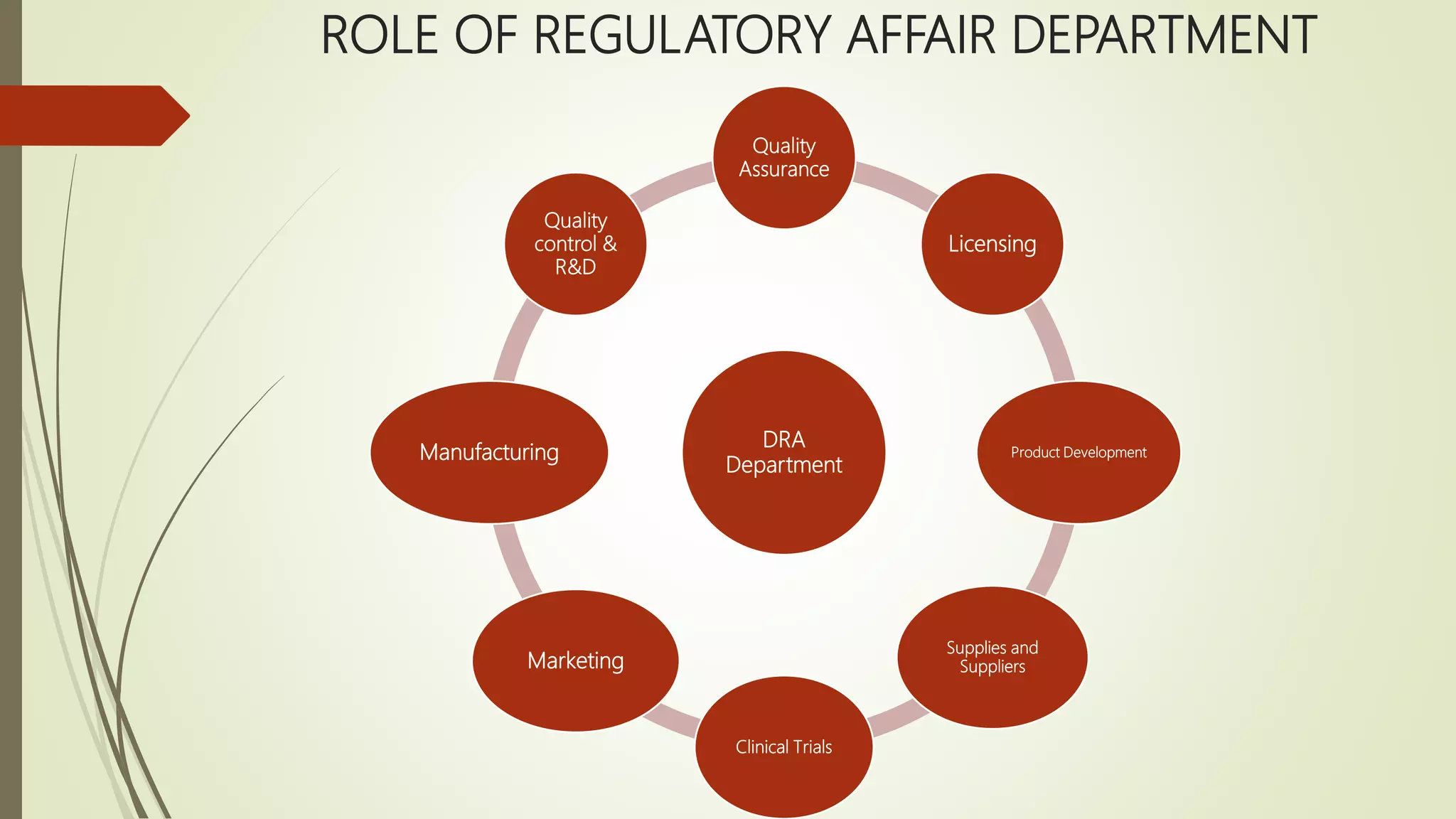
Regulatory affairs professionals play a crucial role in ensuring pharmaceutical products meet regulatory standards for safety, efficacy and quality. They provide strategic guidance to various departments on regulatory requirements and work to obtain approvals from regulatory authorities. Key responsibilities include filing product registrations, tracking legal changes, and facilitating clinical trials and product approvals. Regulatory affairs has become increasingly important given historical issues that prompted stronger drug regulations to protect public health.