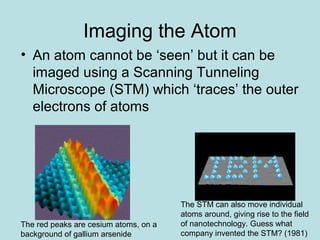
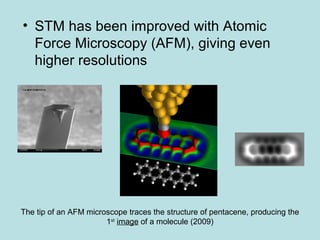
- Early atomic theory proposed that all things are made up of tiny particles called atoms that are constantly in motion, attracting each other at distances but repelling when squeezed together. This single sentence contains a vast amount of information about the structure of matter.
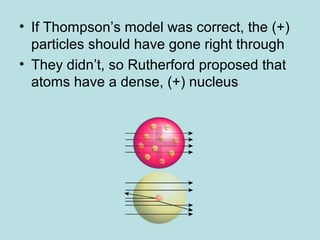
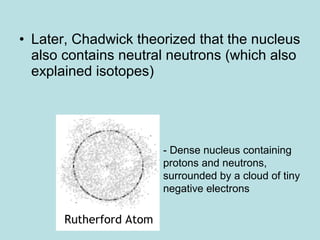
- The atomic model has been improved over time through experiments and evidence. Rutherford's gold foil experiment in 1911 led him to propose that atoms have a small, dense nucleus surrounded by orbiting electrons. Later additions such as Chadwick's discovery of neutrons further refined the model.
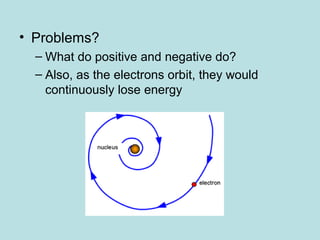
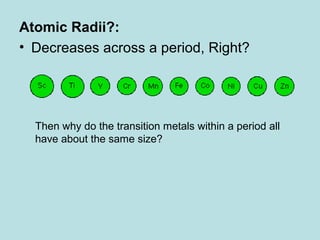
- Continued anomalies and new evidence have prompted further refinement of the atomic model. The current model resolves issues like how electrons can orbit the nucleus without spiraling in. Science works through an