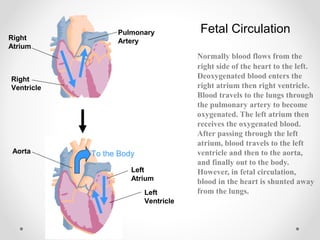
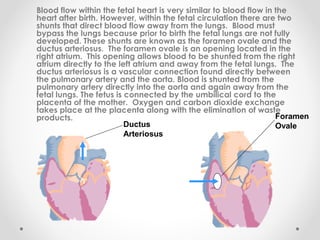
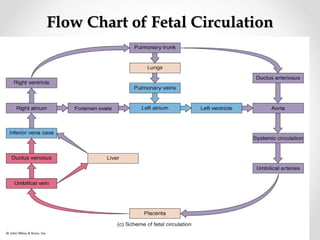
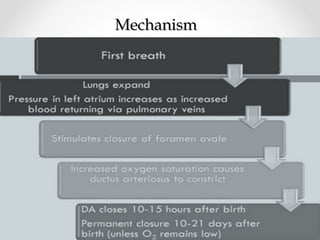
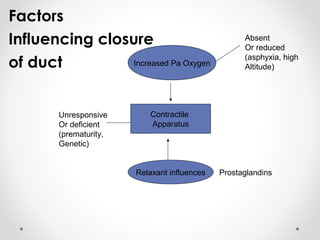
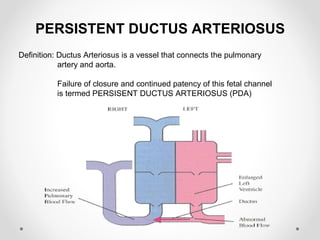
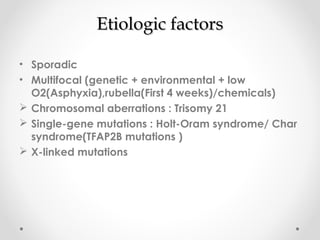
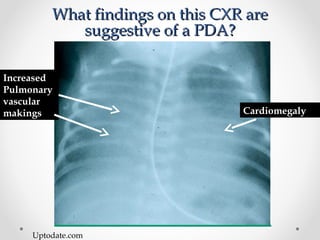
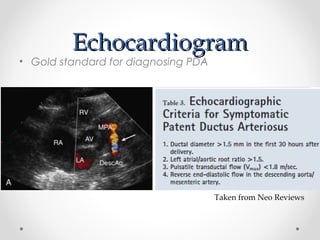
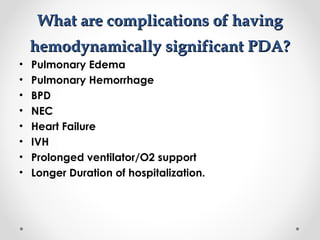
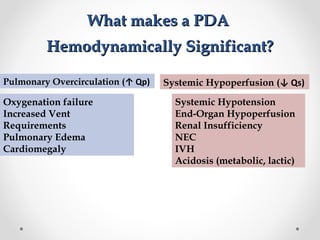
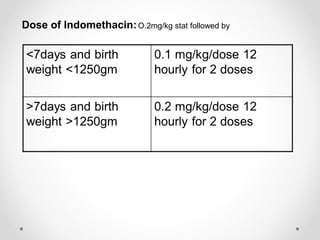
This infant is a 5 day old 25 week preemie with worsening respiratory distress requiring increased oxygen and ventilator support. On exam, the infant has a systolic murmur and signs of increased cardiac output including bounding pulses. An echocardiogram is needed to confirm a diagnosis of a hemodynamically significant patent ductus arteriosus (PDA), which could be contributing to the respiratory distress. Treatment options for a large PDA include supportive care, medications like indomethacin or ibuprofen to close the ductus, or surgery.