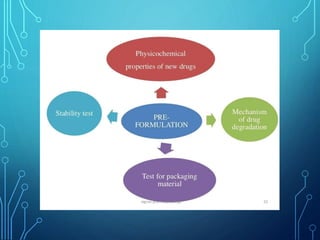
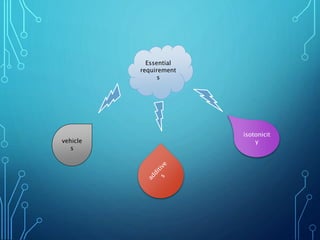
Parenteral products are sterile preparations intended for administration through one or more layers of skin or mucous membranes. They include injections, infusions, powders for injection, and implants. Injections contain sterile solutions, while infusions are isotonic with blood. Single dose preparations are used once while multi-dose preparations contain preservatives. Parenterals must use suitable vehicles like water for injection, be isotonic to prevent hemolysis, and include necessary additives and buffers. They provide advantages of high bioavailability and no risk of missed doses over oral medications.