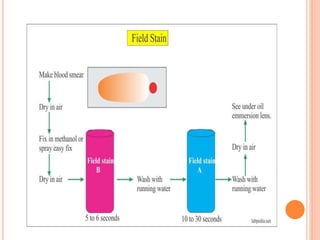
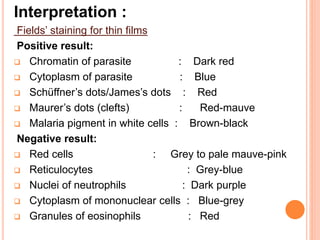
This document describes staining techniques used to identify microorganisms like microsporidia and Plasmodium species. It explains the Calcofluor stain method used to identify microsporidia by staining the chitin in their endospore walls fluorescent blue-white. The Field's stain method used to identify Plasmodium species in blood films is also outlined, noting the staining characteristics of the parasite at different life stages. Lastly, the document discusses using Lugol's iodine to stain protozoan cysts and ova in faecal wet mounts, making their internal structures more visible.