This document discusses the structure of atoms, radioactivity, and uses evidence about radiation and the universe to support the Big Bang theory. It describes:
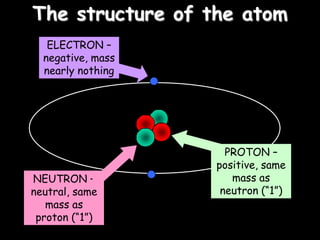
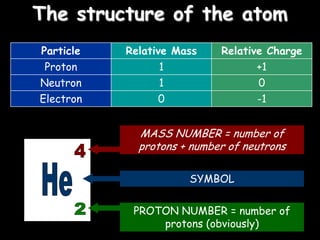
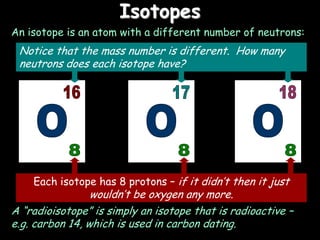
1) The basic structure of atoms including protons, neutrons, electrons, isotopes, and radioisotopes.
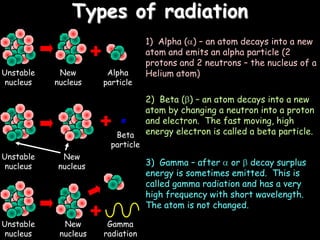
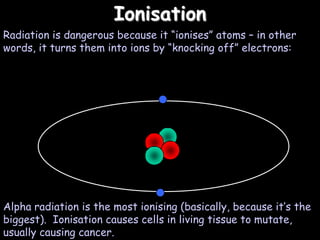
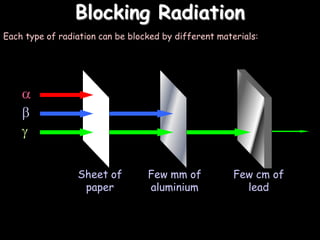
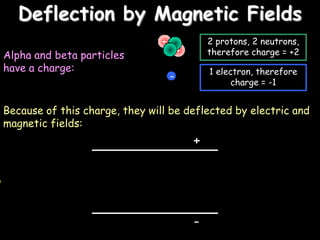
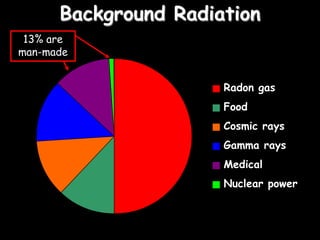
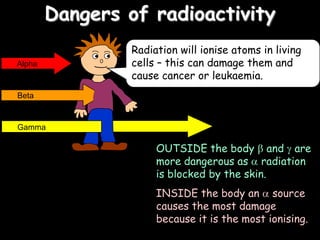
2) Different types of radiation including alpha, beta, and gamma rays and how they are produced and can be blocked.
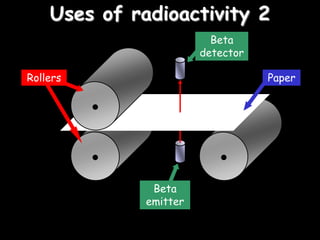
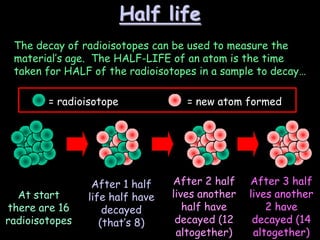
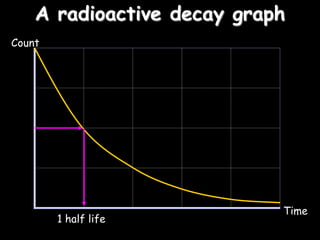
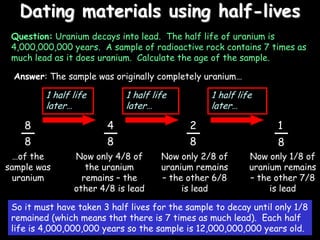
3) How radioisotopes are used in medical tracers and carbon dating to measure material ages based on half-life decay.
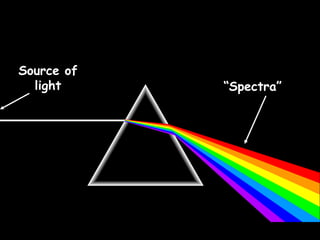
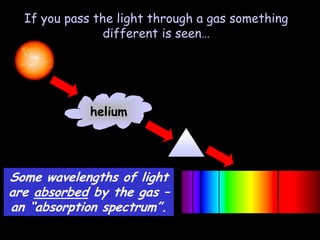
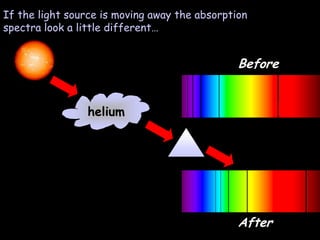
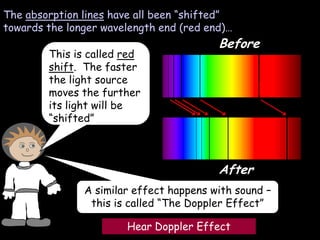
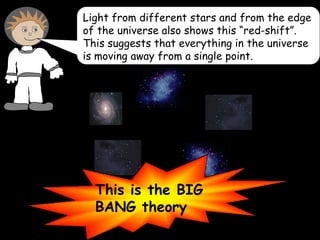
4) Evidence from light spectroscopy showing redshift of spectra from distant galaxies, which suggests everything is moving away from a single point supporting the Big Bang theory of an expanding universe.