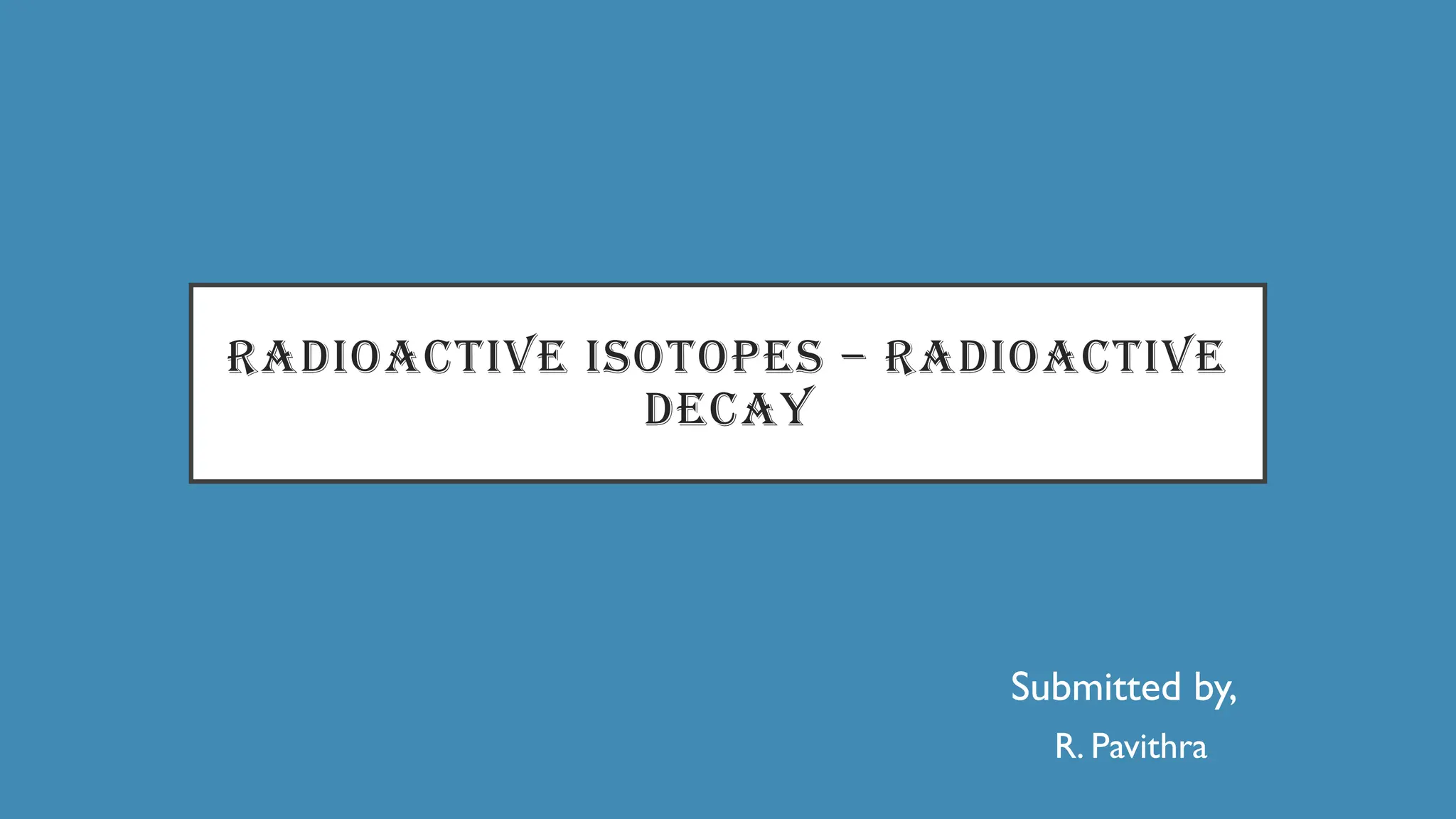
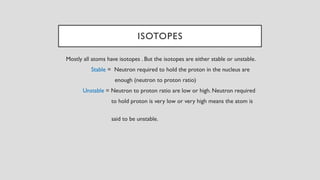
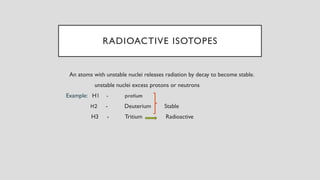
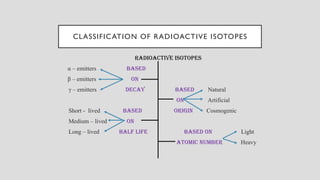
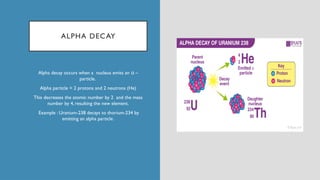
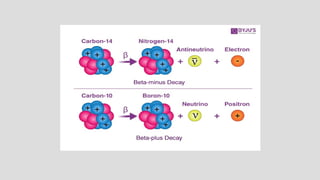
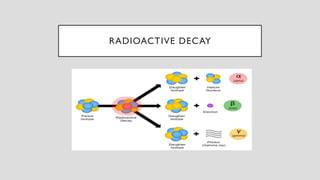
The document discusses radioactive isotopes and their properties, explaining that isotopes are atoms of the same element with the same atomic number but different mass numbers. It details the process of radioactive decay, types of decay (alpha, beta, and gamma), and classifications of isotopes based on various criteria such as stability and atomic number. Additionally, it highlights the applications of radioactive isotopes in fields like radiocarbon dating and smoke detection.