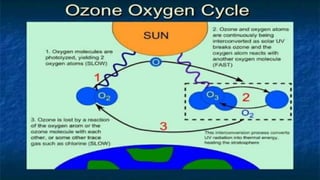
The document summarizes information about ozone layer depletion. The main causes of ozone layer depletion are chlorofluorocarbons (CFCs) released by products like aerosols and refrigerants, as well as rocket launches. CFCs release chlorine atoms that react with and destroy ozone. Effects of ozone layer depletion include increased skin cancer and eye damage for humans, and problems for plants. Prevention efforts include reducing CFC emissions, saving energy, and increasing awareness. The 2019 ozone hole over Antarctica was the smallest on record due to abnormal weather patterns.