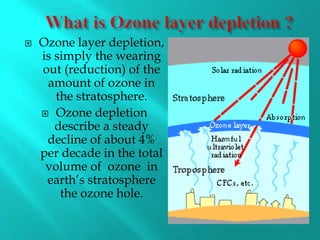
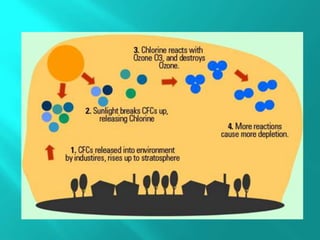
Ozone is a naturally occurring gas made of three oxygen atoms, primarily found in the stratosphere where it forms the ozone layer that absorbs most of the sun’s ultraviolet radiation. Discovered in 1913, the ozone layer is experiencing depletion at a rate of approximately 4% per decade, mainly due to chlorine-containing substances like chlorofluorocarbons (CFCs) from various industrial and consumer products. Ozone depletion has adverse effects on human health, aquatic life, and the environment, leading to increased risks such as eye diseases, skin cancer, and developmental issues in marine species.