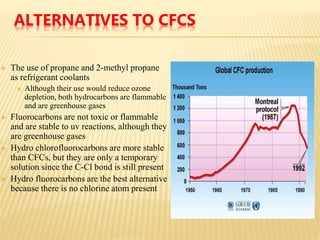
Ozone exists naturally in two layers of the atmosphere. Ground-level ozone is a pollutant but the ozone layer in the stratosphere protects the Earth from harmful UV rays. The ozone layer was depleted by CFCs but this was addressed by the Montreal Protocol which phased out CFC production, allowing the ozone layer to recover over time. Continued monitoring is needed to understand impacts of ozone depletion and climate change.