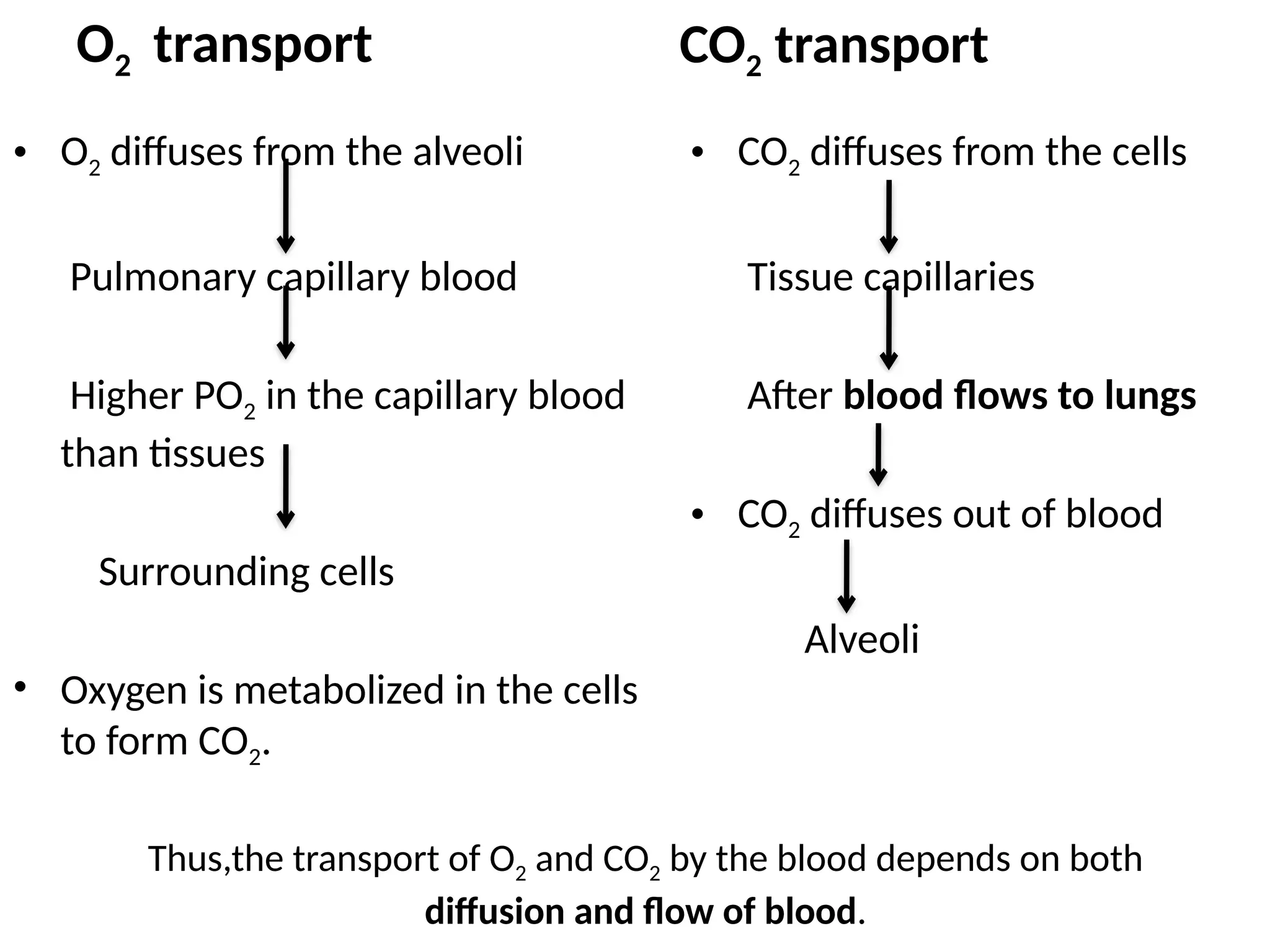
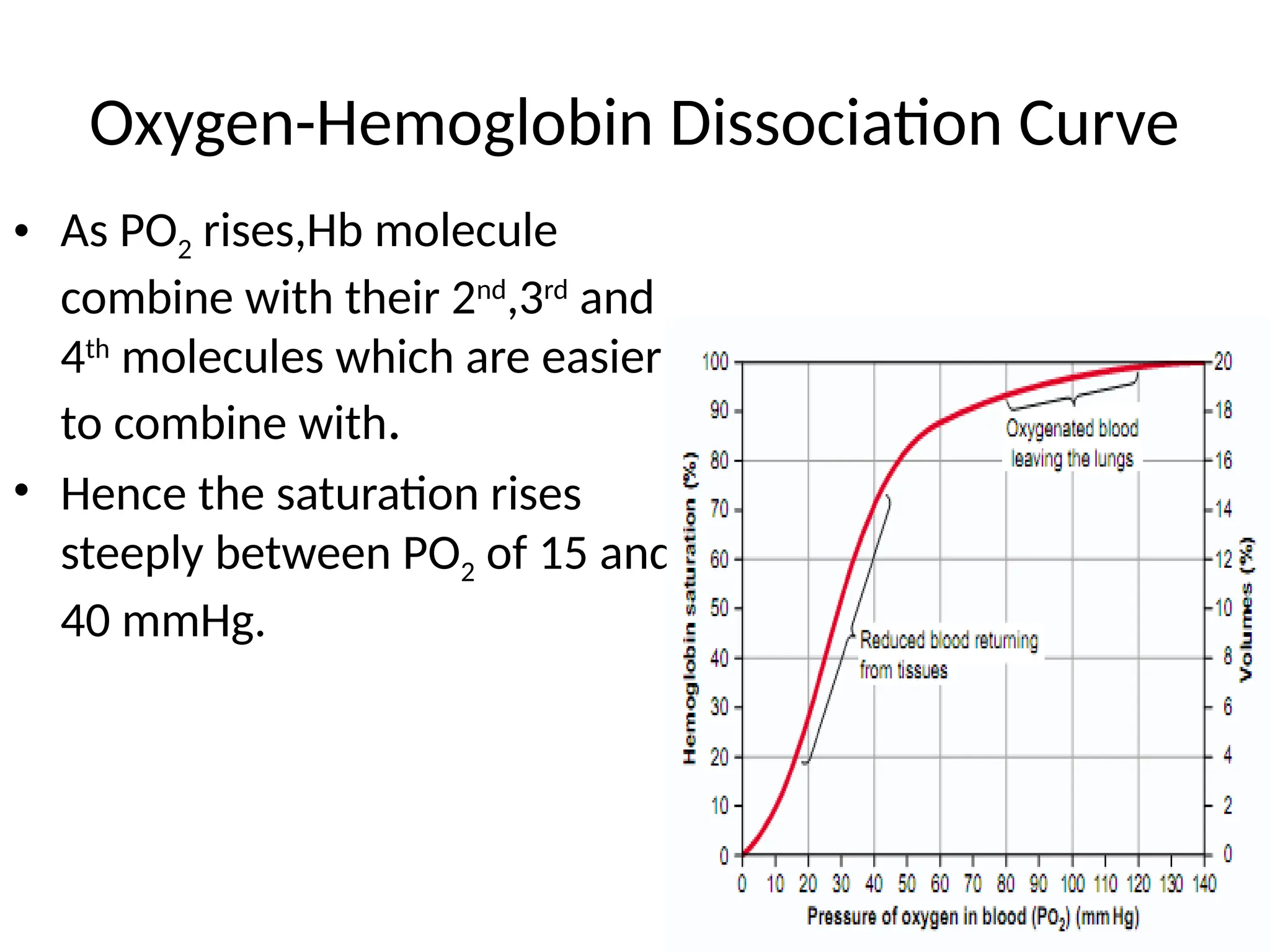
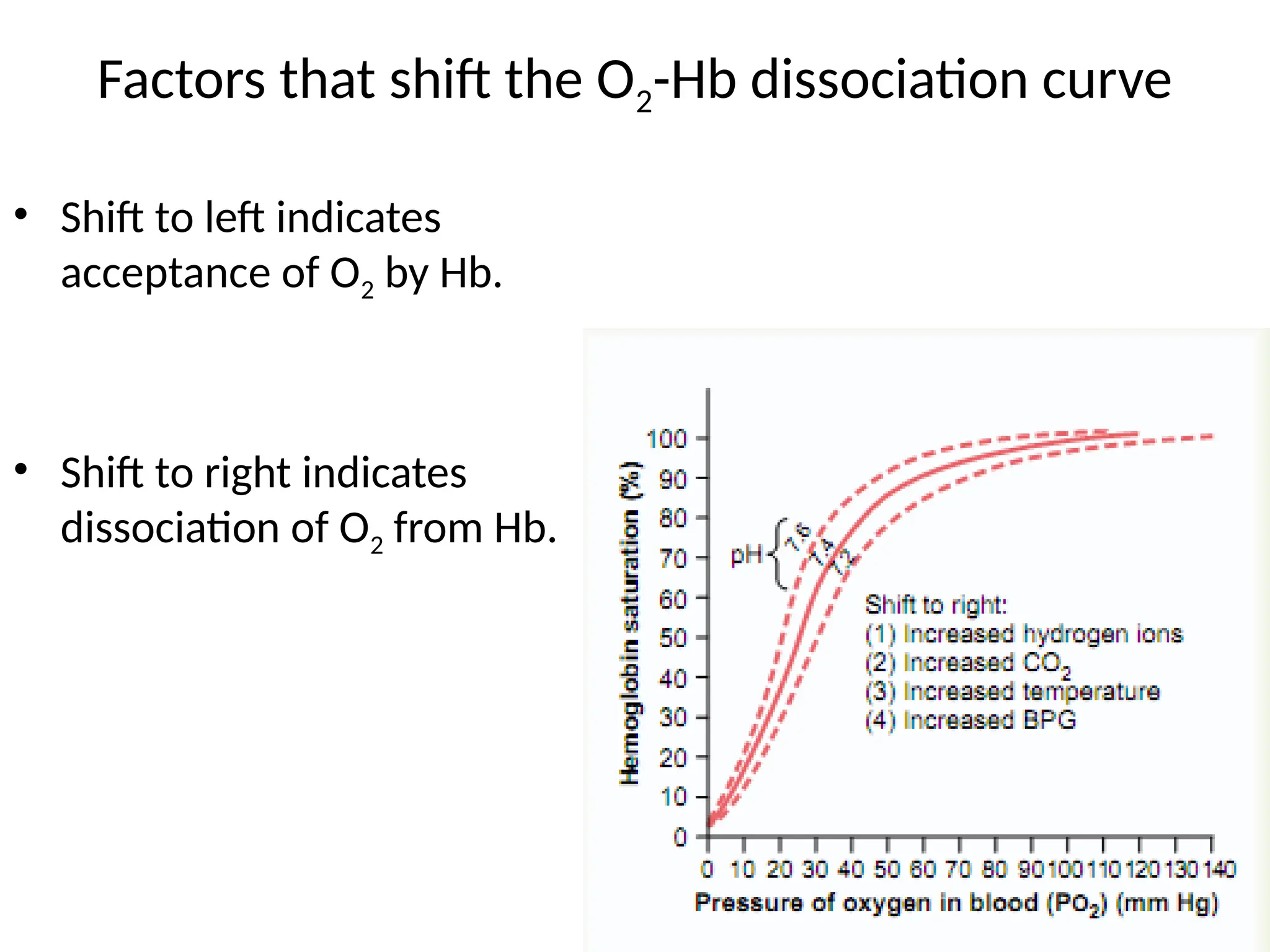
The document explains the transport of oxygen (O2) and carbon dioxide (CO2) in blood and tissue fluids, emphasizing the role of partial pressure differences and diffusion. O2 is primarily transported by hemoglobin in red blood cells, while CO2 is carried in different forms, including dissolved state and bicarbonate ions. The document also discusses the oxygen-hemoglobin dissociation curve, the Bohr and Haldane effects, and factors influencing gas transport in the body.