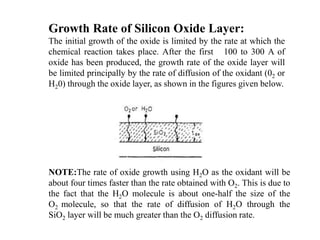
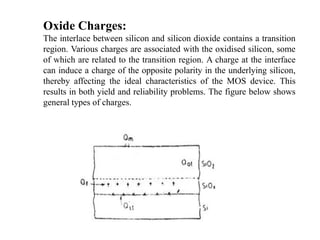
This document discusses oxidation growth kinetics in integrated circuit fabrication. It describes how silicon dioxide is formed through thermal oxidation of silicon wafers in oxygen or water vapor. Silicon dioxide is important in IC fabrication as it can act as an insulator, gate electrode in MOS devices, and provide electrical isolation. The growth rate of silicon dioxide is initially limited by the chemical reaction rate, then by the diffusion rate of oxygen or water molecules through the silicon dioxide layer. Impurities and processing parameters like temperature and exposure time are critical factors that influence the oxidation growth rate.