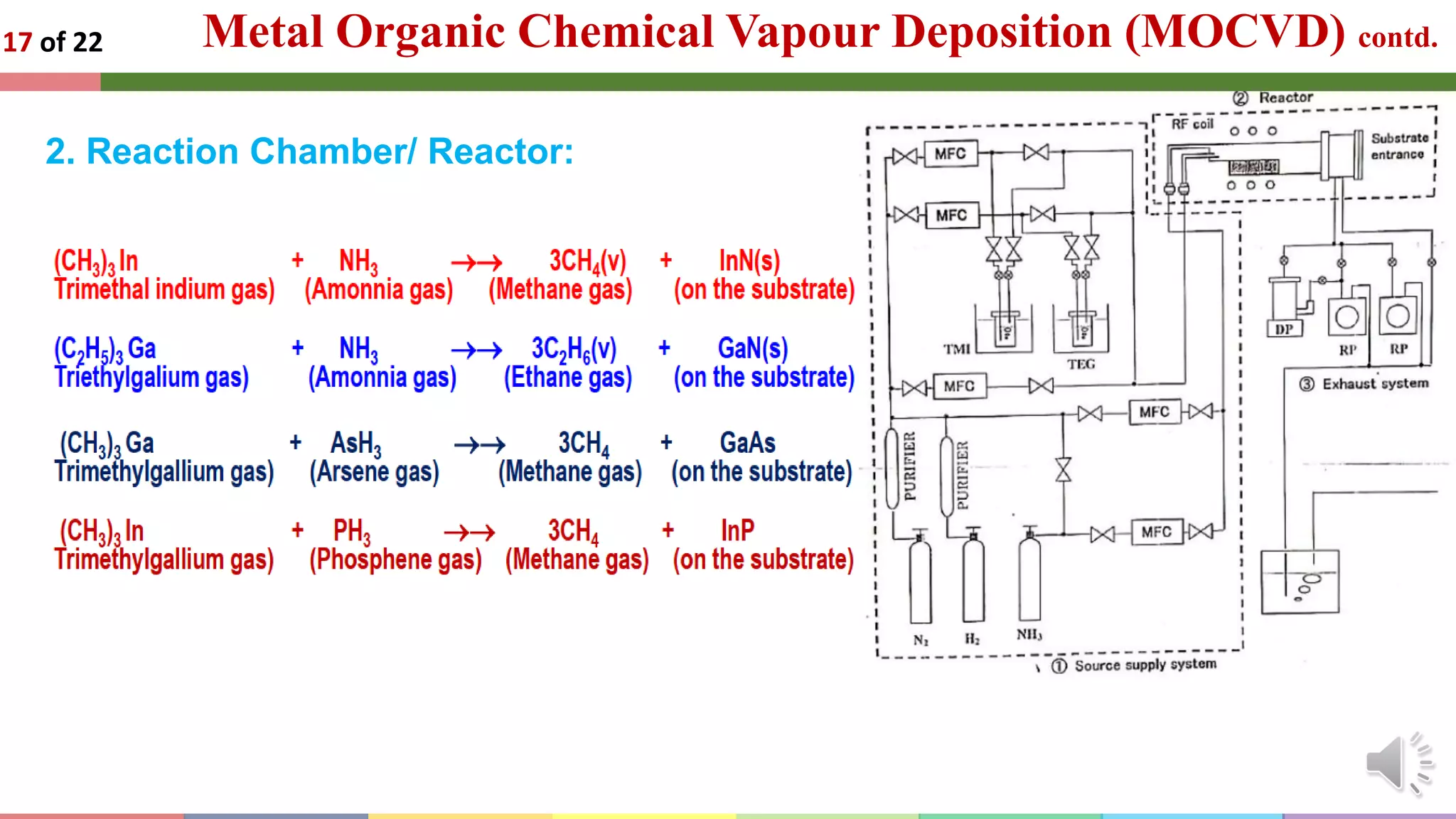
The document presents an introduction to the epitaxial growth of semiconductor materials, covering its mechanisms, methods, and applications. It details different deposition techniques such as liquid phase epitaxy, molecular beam epitaxy, and metal organic chemical vapor deposition, highlighting their advantages and specific uses in electronic devices. The content emphasizes the importance of epitaxial growth in creating high-quality semiconductor layers essential for applications like lasers, LEDs, and transistors.