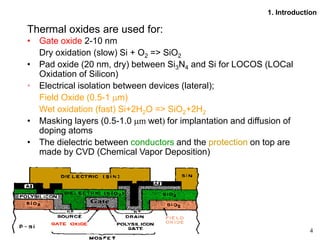
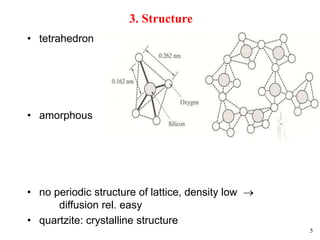
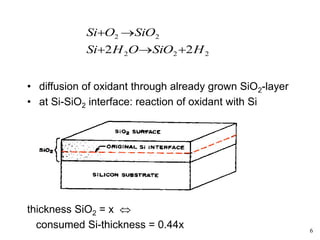
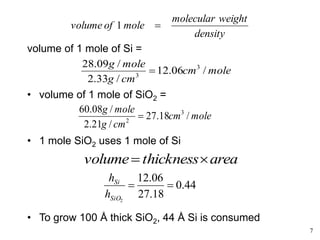
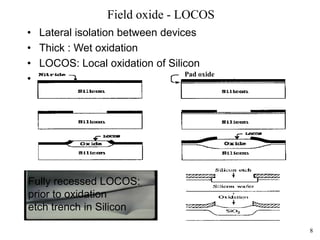
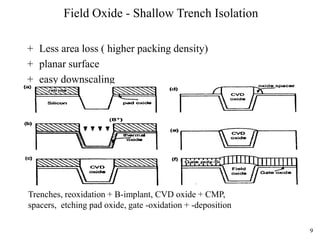
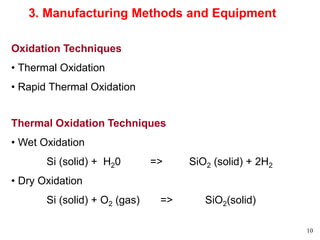
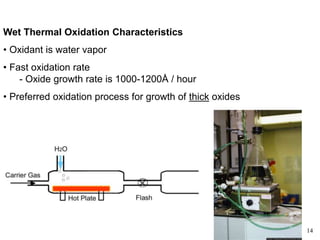
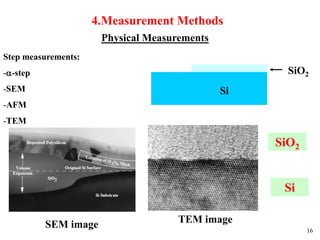
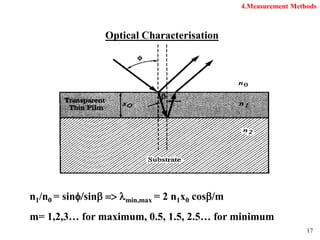
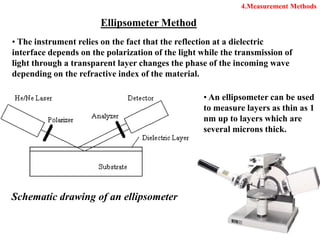
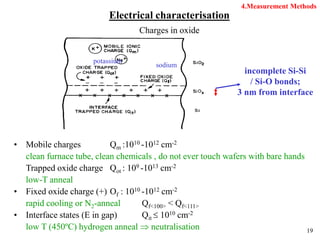
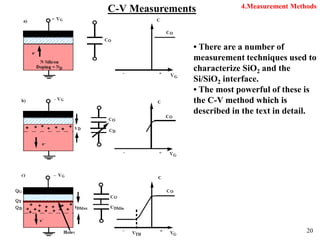
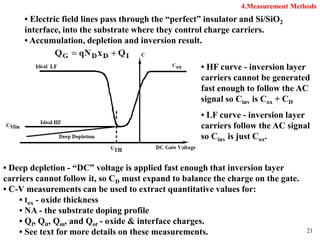
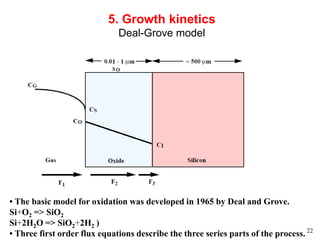
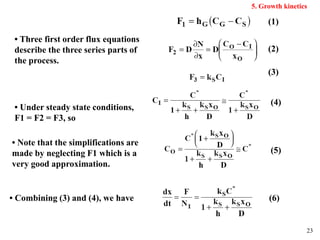
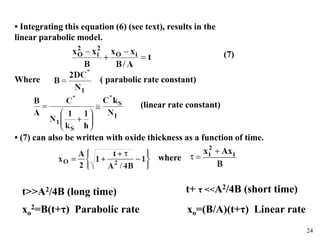
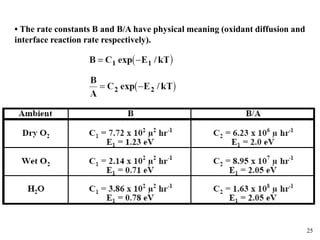
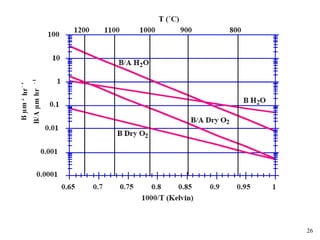
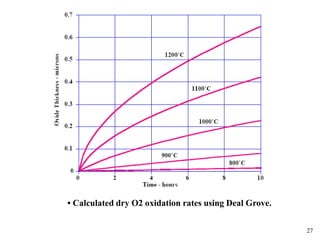
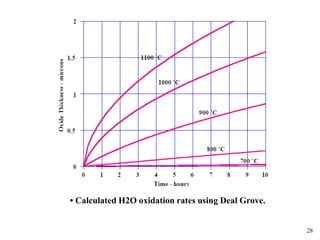
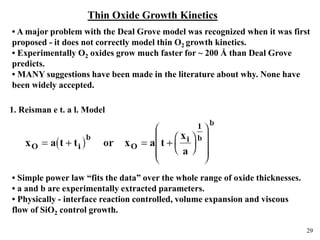
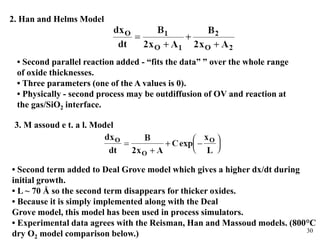
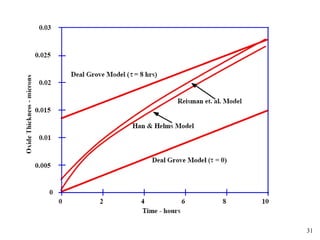
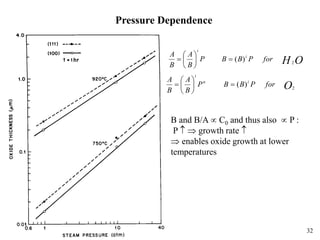
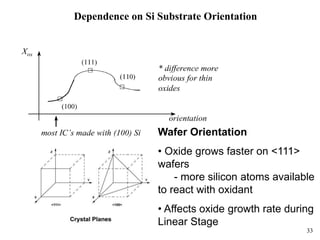
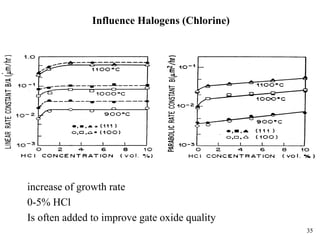
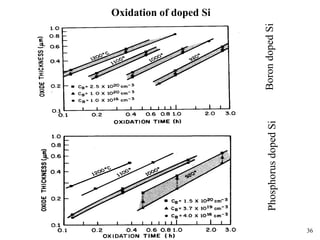
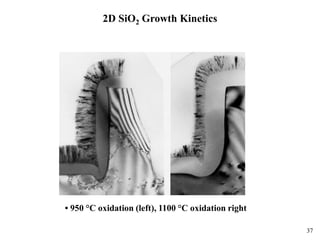
Thermal oxidation of silicon is used to form gate oxides and isolation oxides in integrated circuits. The growth of silicon dioxide (SiO2) on silicon wafers follows the Deal-Grove model of diffusion-limited parabolic growth, with modifications for thin oxide growth in dry oxygen. Measurement techniques like ellipsometry and C-V profiling characterize oxide thickness and quality by measuring fixed charges, interface states, and mobile ions. Advances aim to reduce defects at the Si-SiO2 interface through techniques like nitridation and fluorine incorporation.