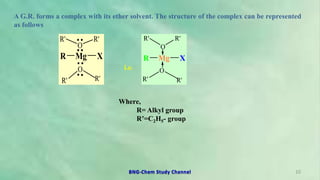
1. Organomagnesium compounds, known as Grignard reagents, were discovered in 1900 by Victor Grignard for which he received the Nobel Prize in 1912.
2. Grignard reagents are prepared by reacting an organic halide with magnesium metal turnings in dry diethyl ether. Common organic halides used are alkyl halides and aryl halides.
3. In the laboratory, Grignard reagents are prepared by adding an alkyl halide dissolved in diethyl ether to a flask containing magnesium turnings and diethyl ether under reflux. A crystal of iodine is sometimes added as a catalyst to initiate the reaction.
![1
B. Sc. Third Year
Semester-V
1
CH-02:- Module-1 : OMC-Introduction and A] Organomagnesium Compounds
Dr. B. N. Gawade
M. Sc. CSIR-UGC-NET (JRF), Ph. D.
Assistant Professor
Department of Chemistry
Anandrao Dhonde Alias Babaji Mahavidyalaya, Kada.
Tal. Ashti. Dist. Beed - 414 202, Maharashtra, INDIA
CH-02: Organometallic Compounds
Welcome](https://image.slidesharecdn.com/ch-02-module-1-omc-introductionandaorganomagnesiumcompounds-211221101639/75/CH-02-Module-1-OMC-Introduction-and-a-Organomagnesium-Compounds-1-2048.jpg)



![Organomagnesium halides R-Mg-X were discovered by the French chemist victor Grignard in
1900. Grignard received the Nobel Prize for his discovery in 1912 and organomagnesium halides
are called Grignard reagents in his honour.
Discovered by Victor Grignard in 1900
– Key factors are ethereal solvent
and water-free conditions
Awarded Nobel Prize in 1912
Victor Grignard, 1871–1935, French chemist. He shared the 1912 Nobel Prize in
Chemistry for his work in organic synthesis based on his discovery (1900) of the
Grignard Reagent. He taught at the Univ. of Nancy (1909–19) and at the Univ. of
Lyons (from 1919 until the end of his career). 5
A] ORGANOMAGNESIUM COMPOUNDS
BNG-Chem Study Channel](https://image.slidesharecdn.com/ch-02-module-1-omc-introductionandaorganomagnesiumcompounds-211221101639/85/CH-02-Module-1-OMC-Introduction-and-a-Organomagnesium-Compounds-5-320.jpg)