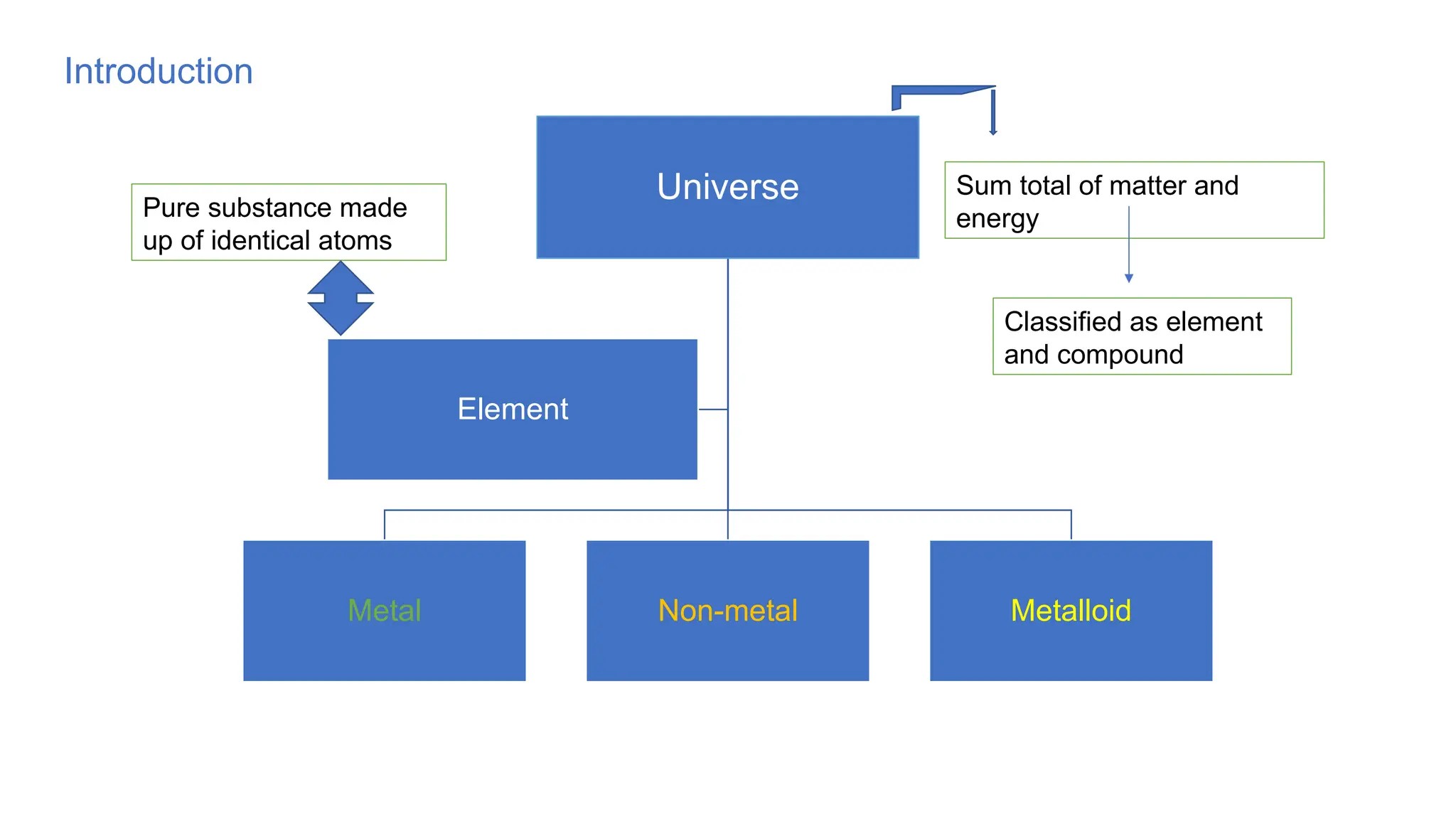
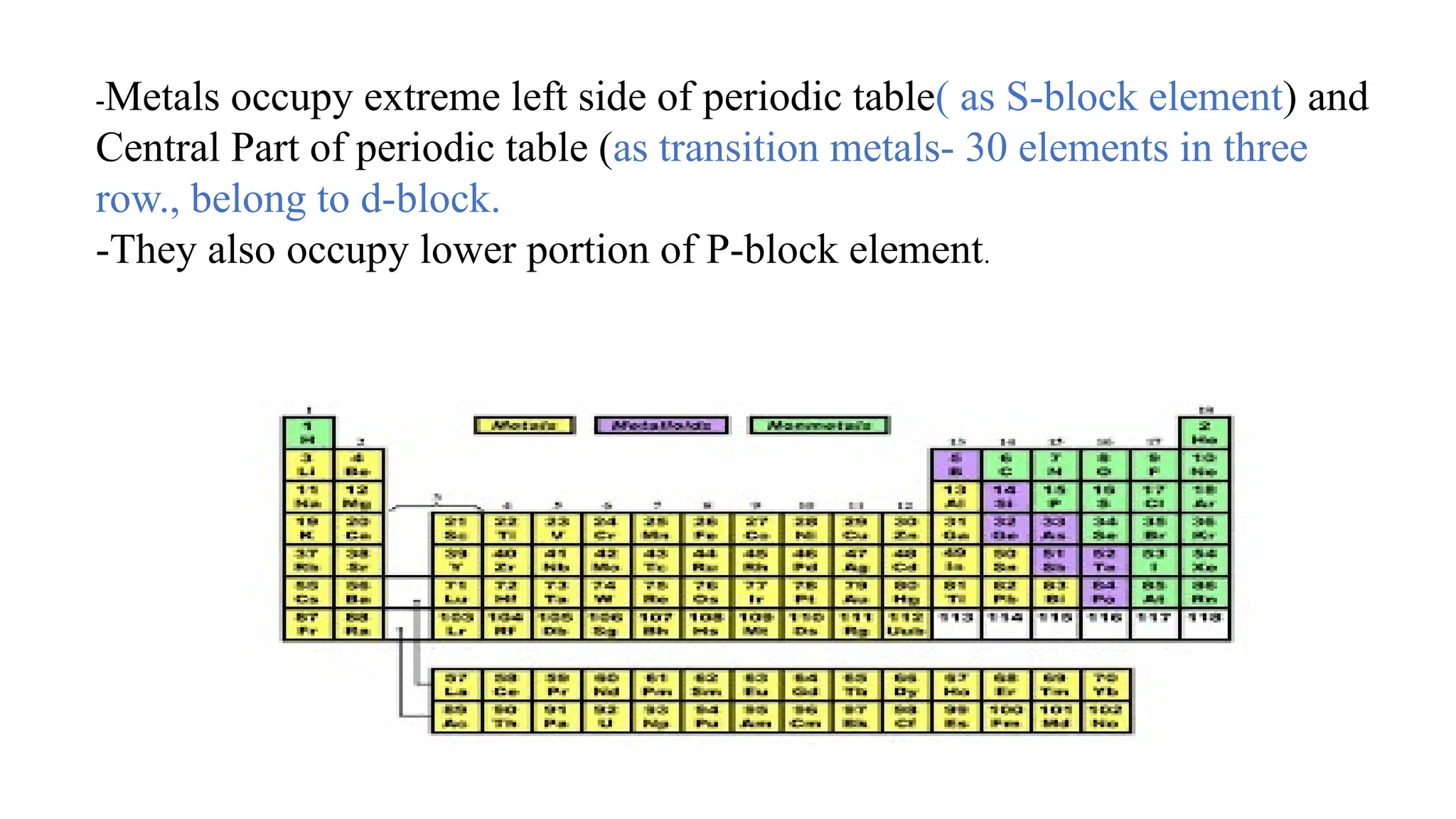
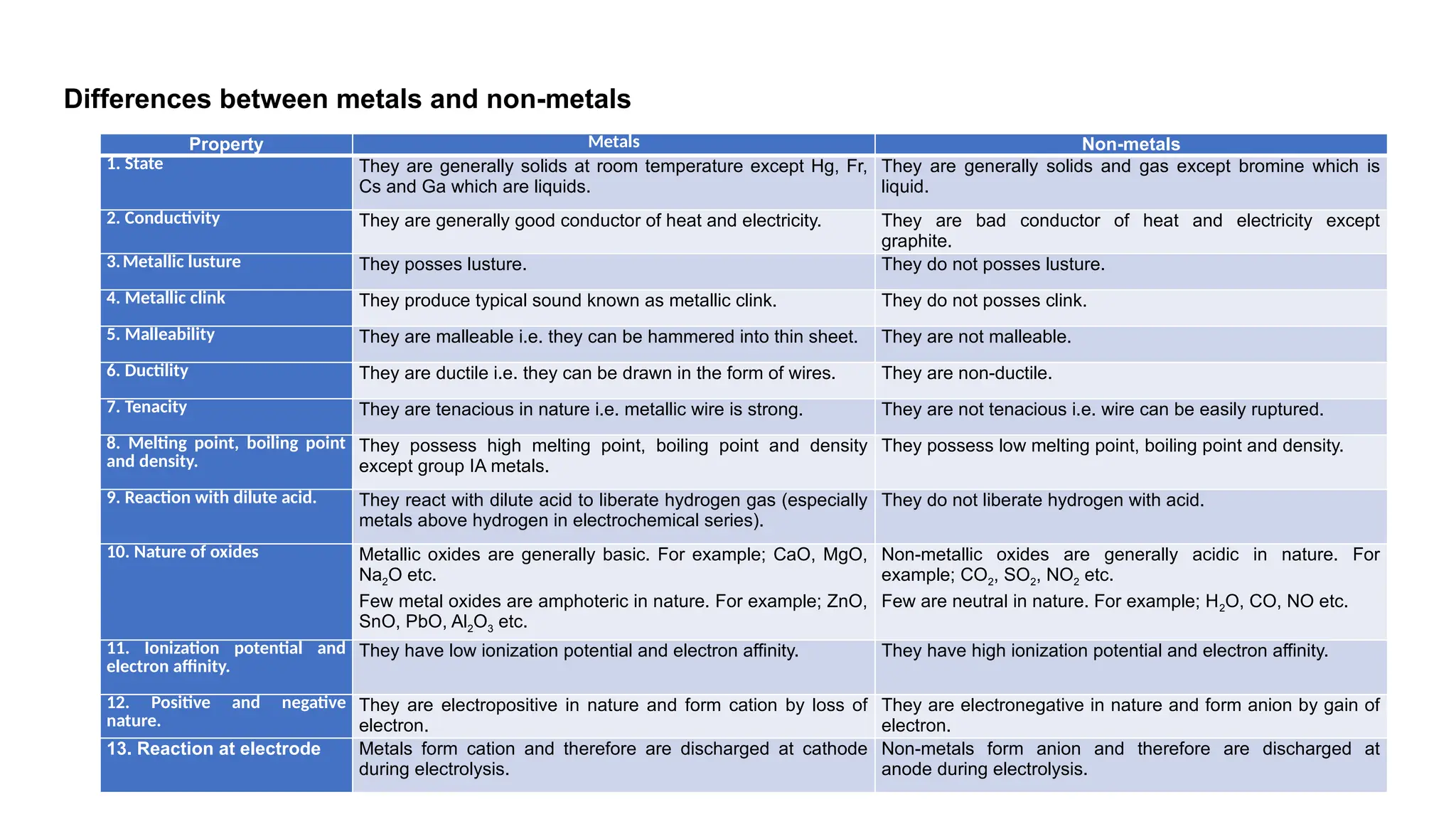
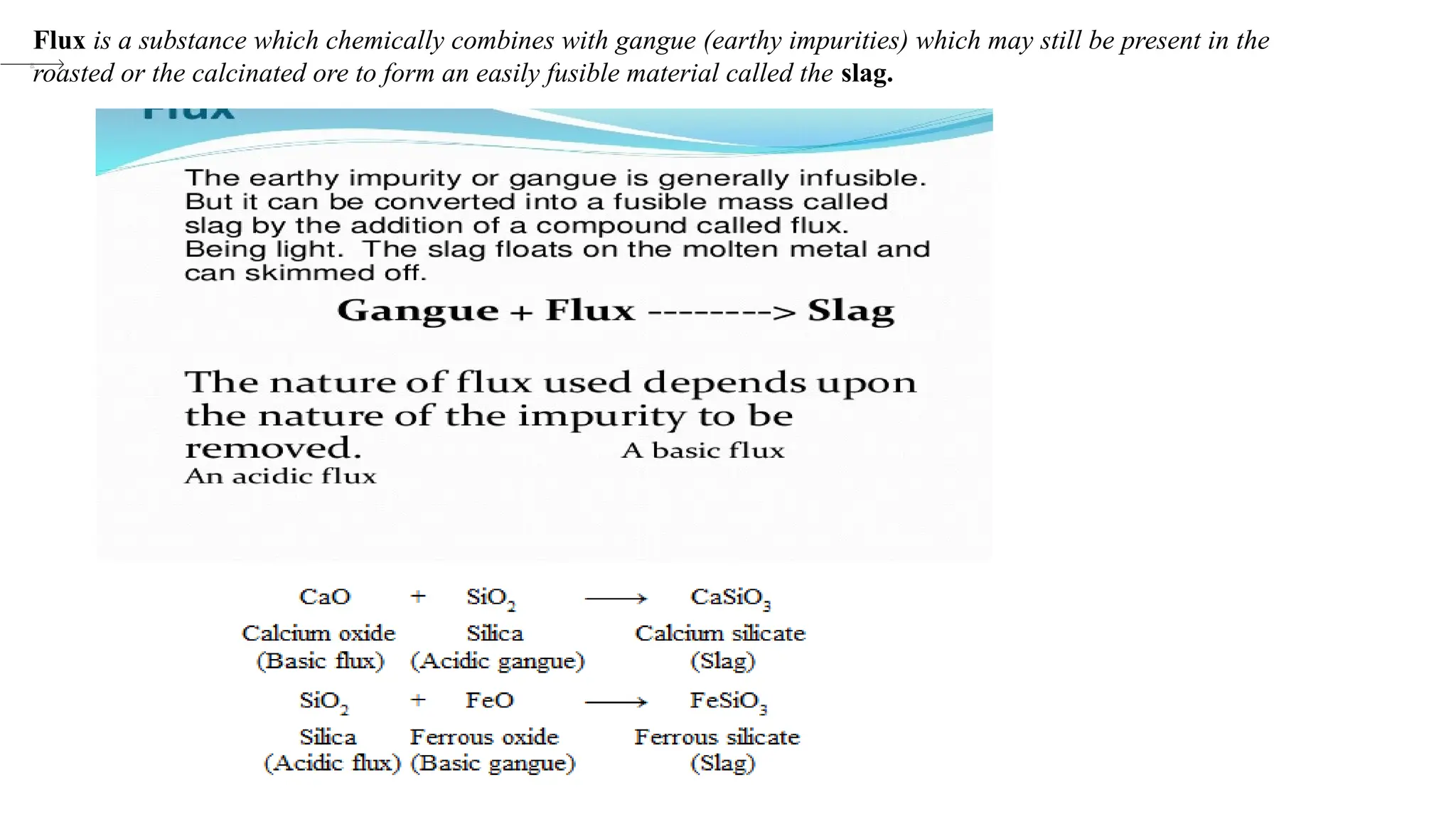
The document outlines the differences between metals, non-metals, and metalloids, including their properties and classifications. It explains the occurrence of elements in native and combined states and provides definitions for various related terms such as ores and alloys. Additionally, it details the characteristics of alloys and amalgams, emphasizing their composition and properties.