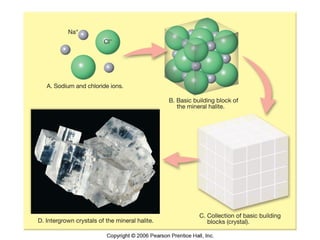
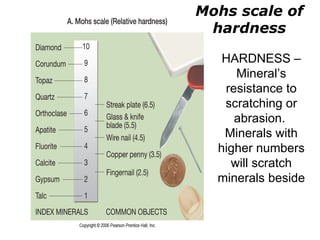
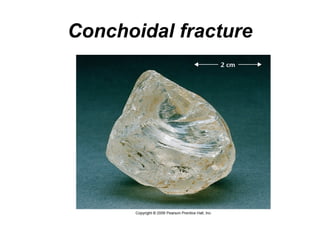
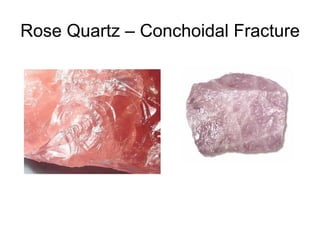
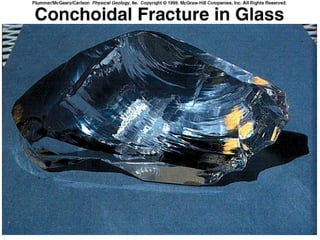
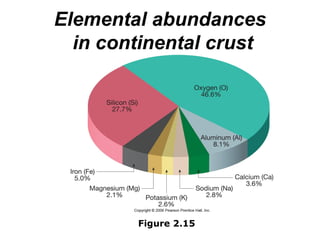
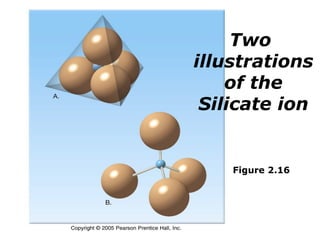
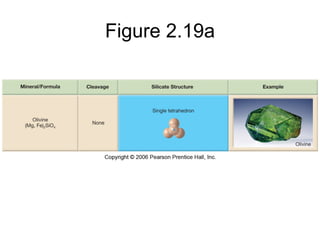
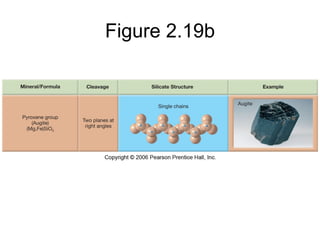
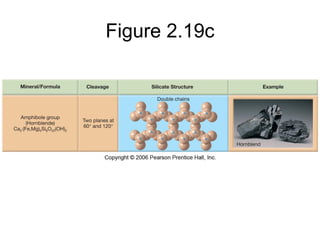
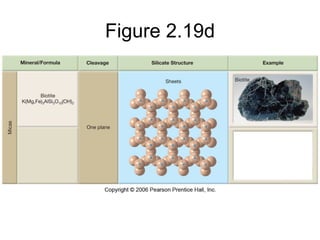
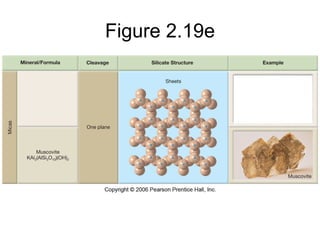
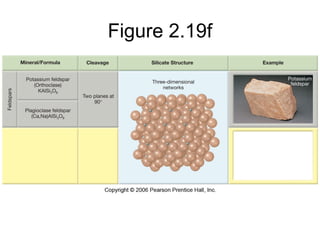
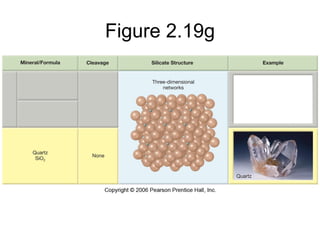
The document provides a comprehensive overview of minerals, defining key concepts such as minerals, rocks, and their physical properties including luster, color, streak, hardness, cleavage, and fracture. It discusses the classification of over 4000 minerals, emphasizing silicates as the most abundant rock-forming minerals, along with important nonsilicate minerals. The text also covers special characteristics used to identify minerals, such as magnetism, reaction to acids, and fluorescence.