Embed presentation
Download as PDF, PPTX



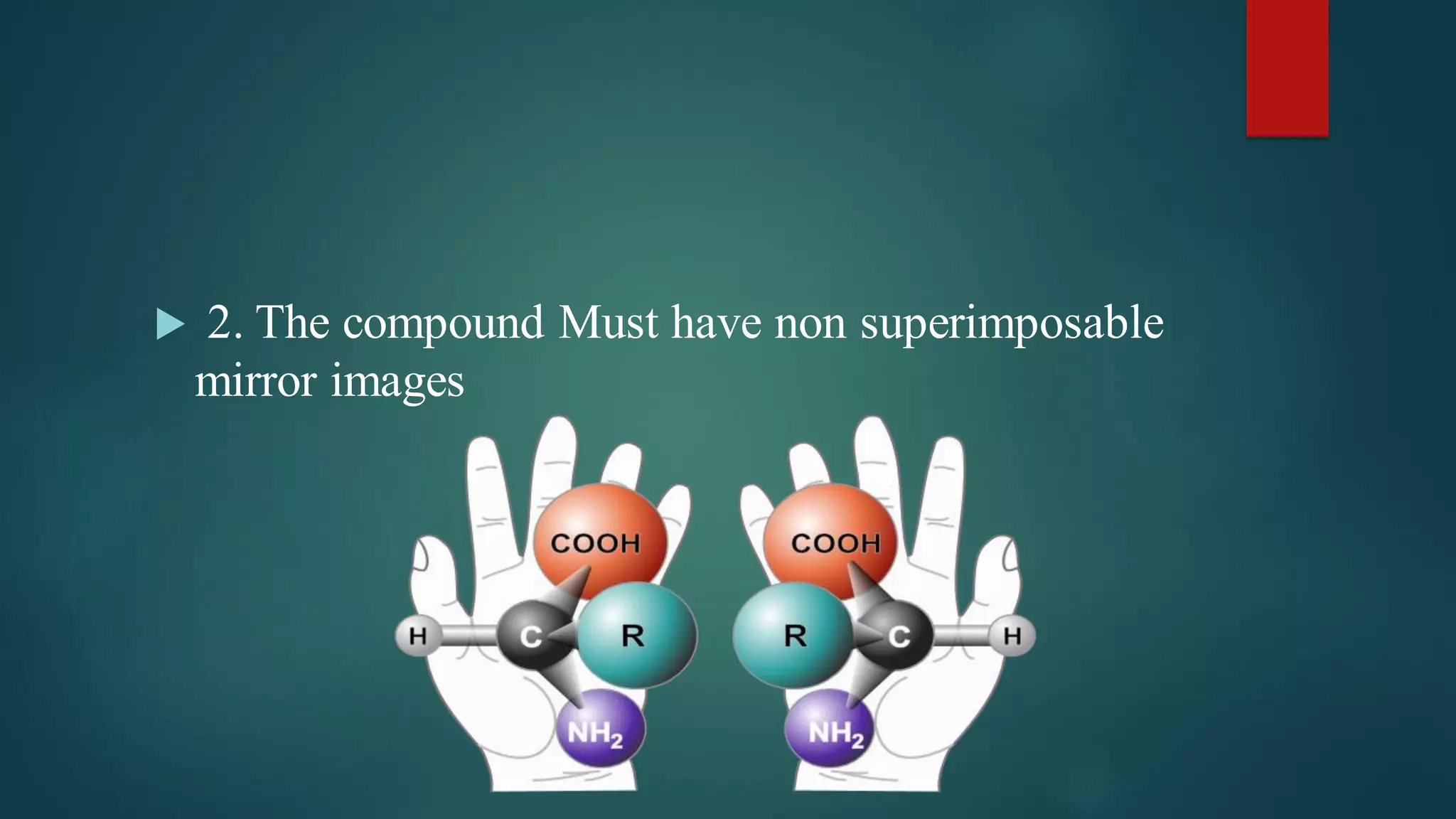

A compound exhibits optical activity if it contains a chiral carbon atom, which is an asymmetric carbon atom bonded to four different atoms or groups. For a compound to be optically active it must have non-superimposable mirror images and cannot contain any symmetry elements like a plane of symmetry, center of symmetry, or axis of symmetry. Optical activity is demonstrated by a compound's ability to rotate the plane of polarized light as it passes through.




