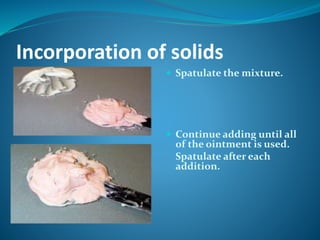
This document defines ointments as semi-solid preparations for application to the skin. It discusses the types of ointments including medicated and non-medicated. It describes the ideal properties of ointments and different bases used to make them, including oleaginous, absorption, water-removable, and water-soluble bases. Methods for preparing ointments by incorporation and fusion are also outlined.