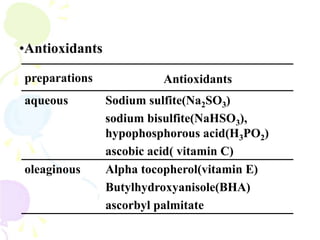
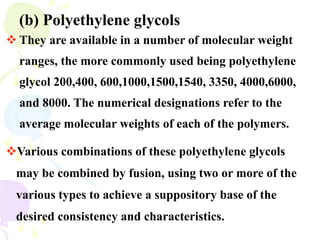
This document discusses semisolid dosage forms, specifically focusing on ointments and ophthalmic ointments. It defines ointments, describes their main components including various bases and adjuvants. It also covers the classification, quality requirements, preparation and quality control of ointments. For ophthalmic ointments, it notes their special preparation under aseptic conditions for application to the eye.