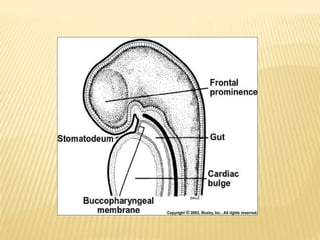
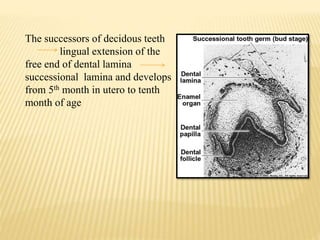
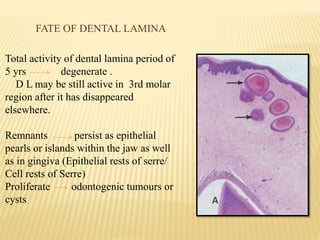
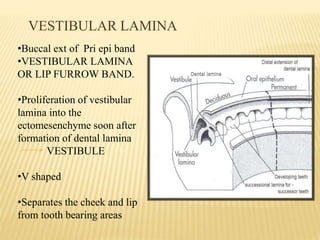
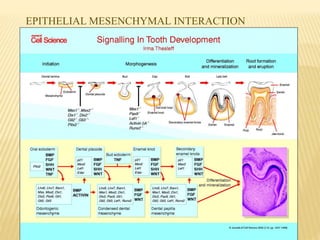
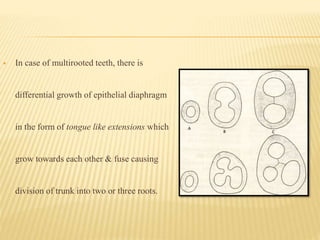
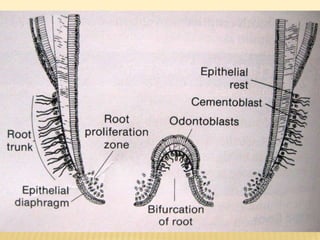
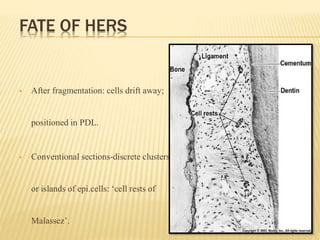
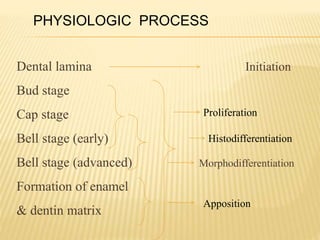
This document provides an overview of odontogenesis, the process of tooth development. It discusses how teeth develop through the interaction of oral epithelial cells and underlying mesenchymal cells. The key stages of tooth development include:
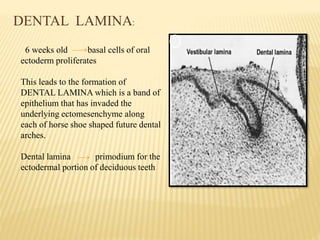
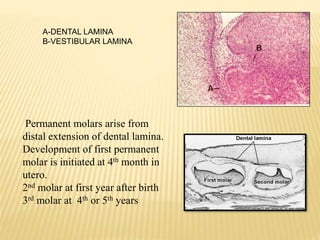
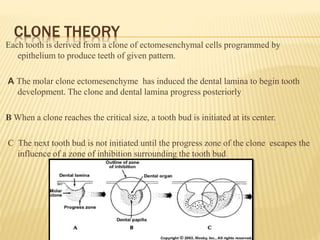
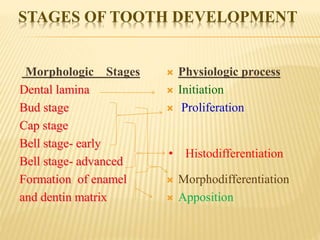
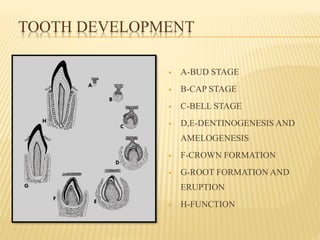
1) Formation of the primary epithelial band and dental lamina, which gives rise to the tooth bud.
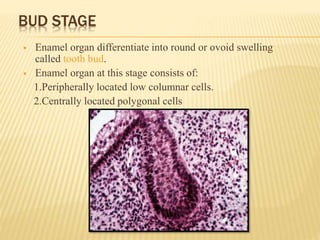
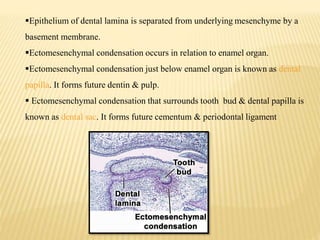
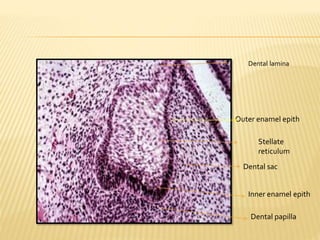
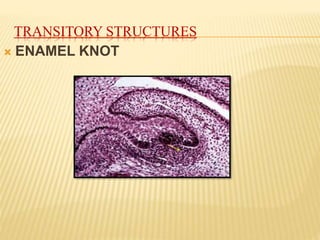
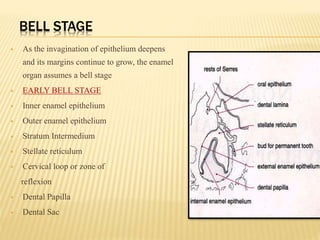
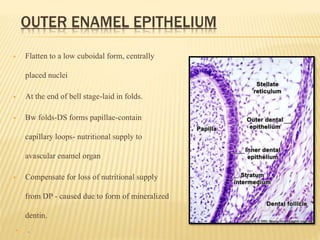
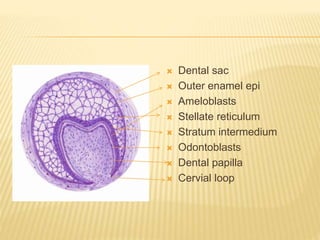
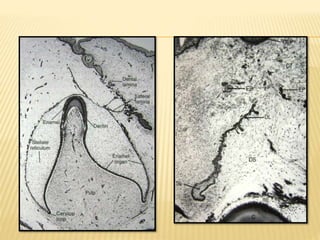
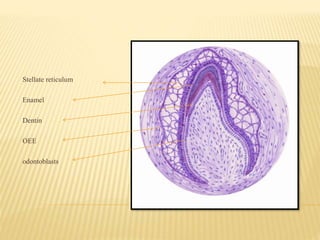
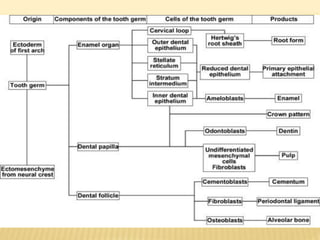
2) Progression through bud, cap, and bell stages as the tooth germ develops through interactions between the enamel organ and dental papilla.
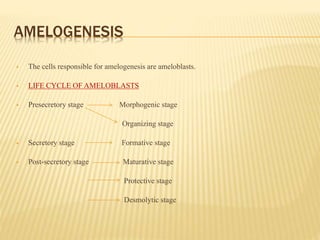
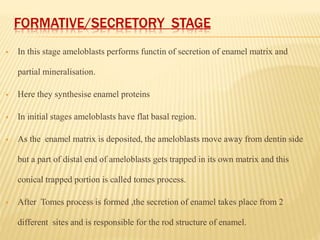
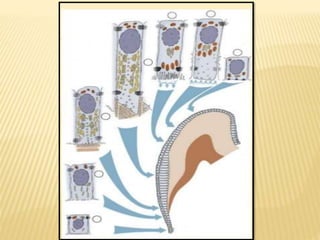
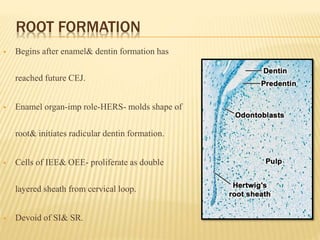
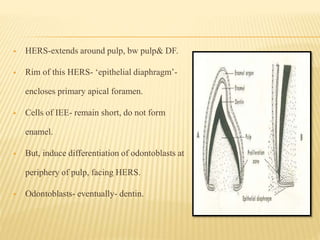
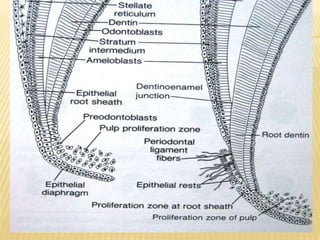
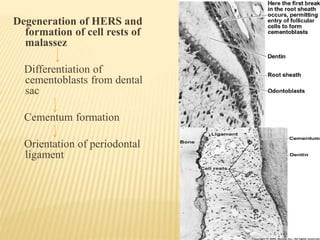
3) Maturation stages involving dentinogenesis, amelogenesis, and root formation that sculpt the tooth shape and structure. A variety of genes regulate this complex process.