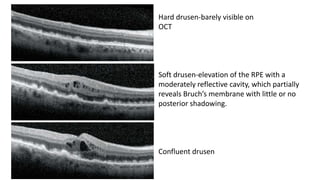
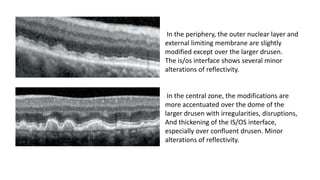
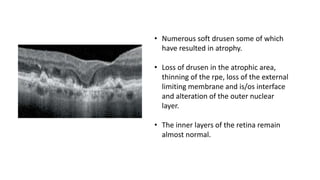
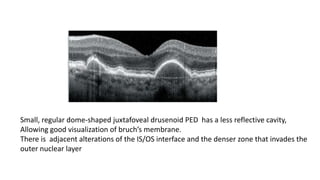
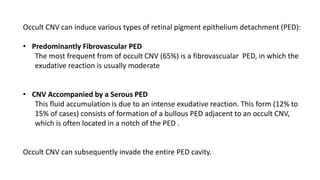
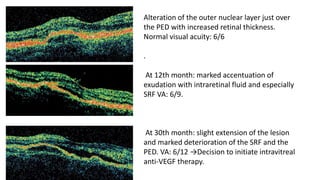
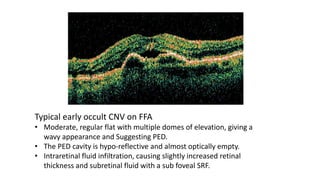
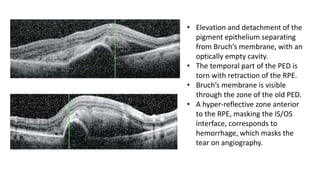
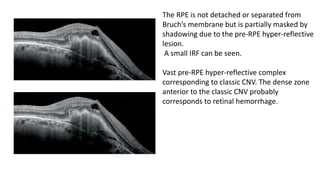
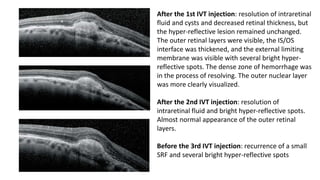
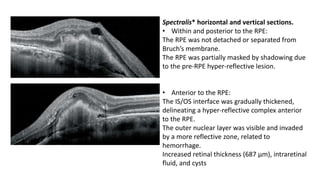
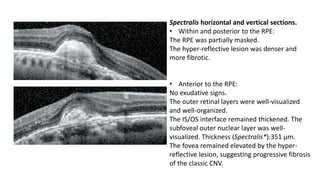
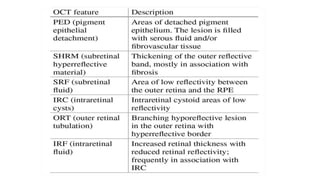
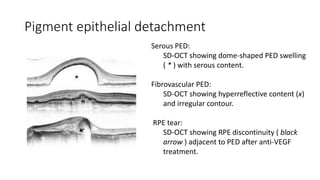
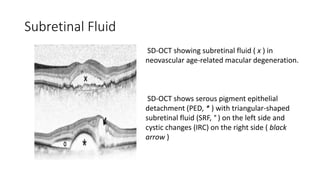
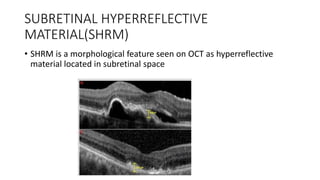
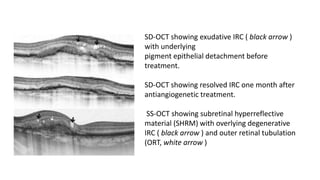
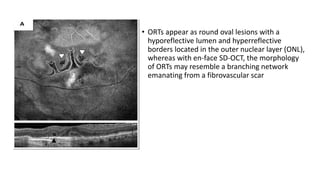
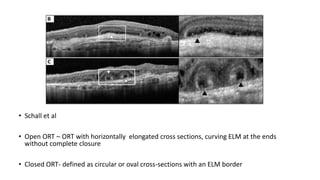
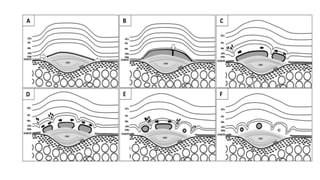
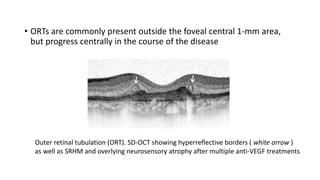
SD-OCT is useful for evaluating pigment epithelial detachments (PEDs), subretinal fluid, and intraretinal features in wet age-related macular degeneration. PEDs can be serous or fibrovascular. Subretinal fluid appears hyporeflective underneath the retina. Persistent subretinal hyperreflective material is associated with worse outcomes. Intraretinal cystoid fluid usually resolves with treatment but can become degenerative. Outer retinal tubulations are structural rearrangements of photoreceptors that develop with significant photoreceptor disruption.