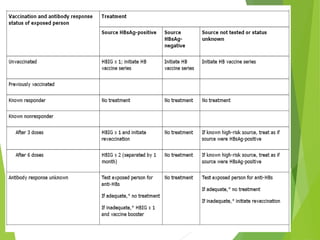
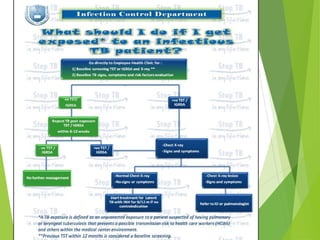
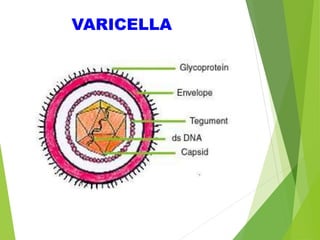
This document summarizes occupational exposures and guidelines for prevention of bloodborne pathogens. It reports that 46.7% of sharp injuries at one hospital were to nursing staff. For HBV, the risk of infection from a sharp exposure to HBsAg-positive blood is 22-31% without vaccination. All healthcare workers should receive the hepatitis B vaccine series. For HCV, the risk of infection from a mucous membrane exposure to an HCV-positive source is 1.8%. For HIV, the risk of infection from a percutaneous exposure to an HIV-positive source is 0.3-0.5%. The document provides post-exposure prophylaxis guidelines for each bloodborne pathogen. It also addresses tuberculosis and varicella