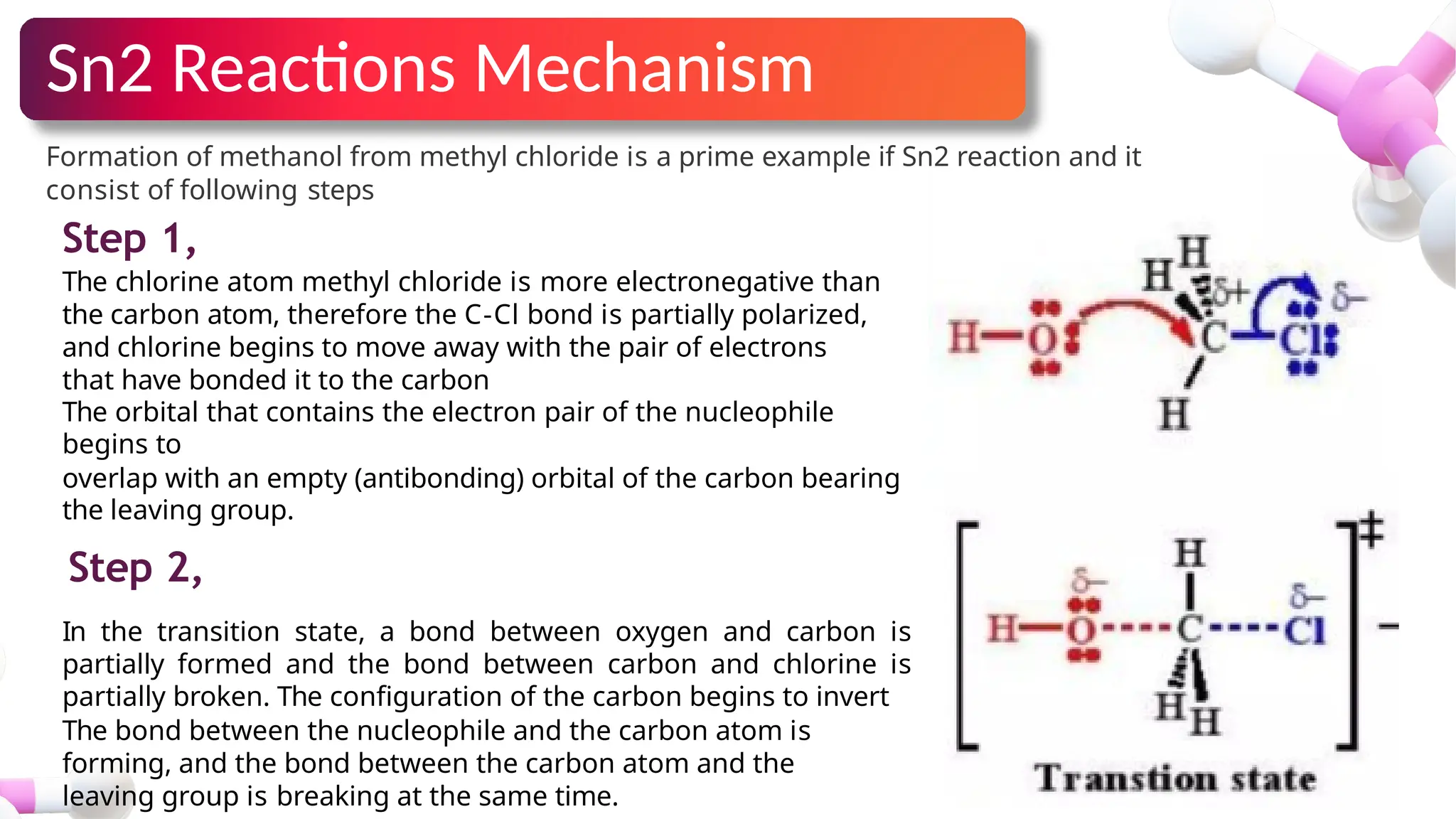
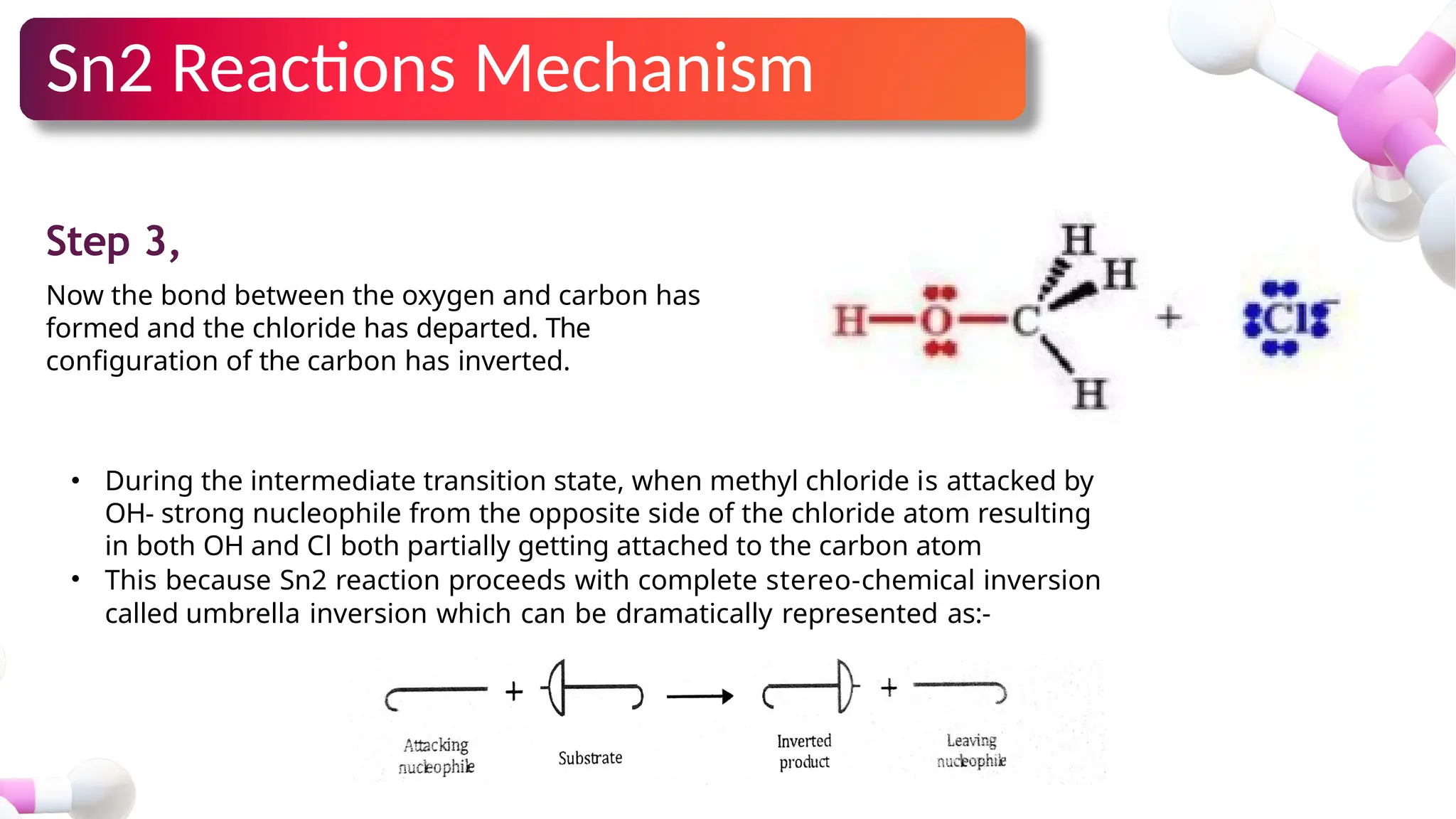
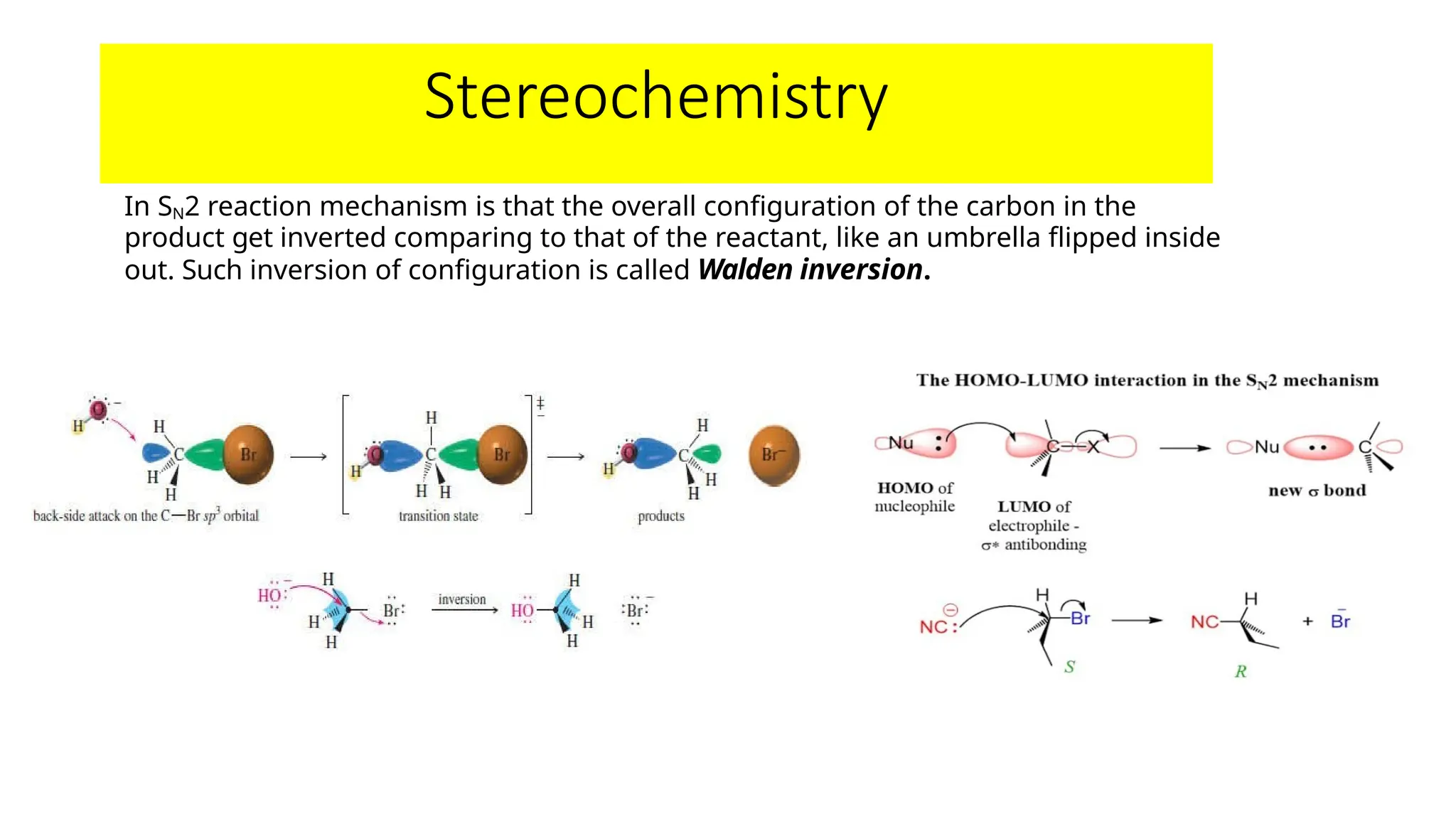
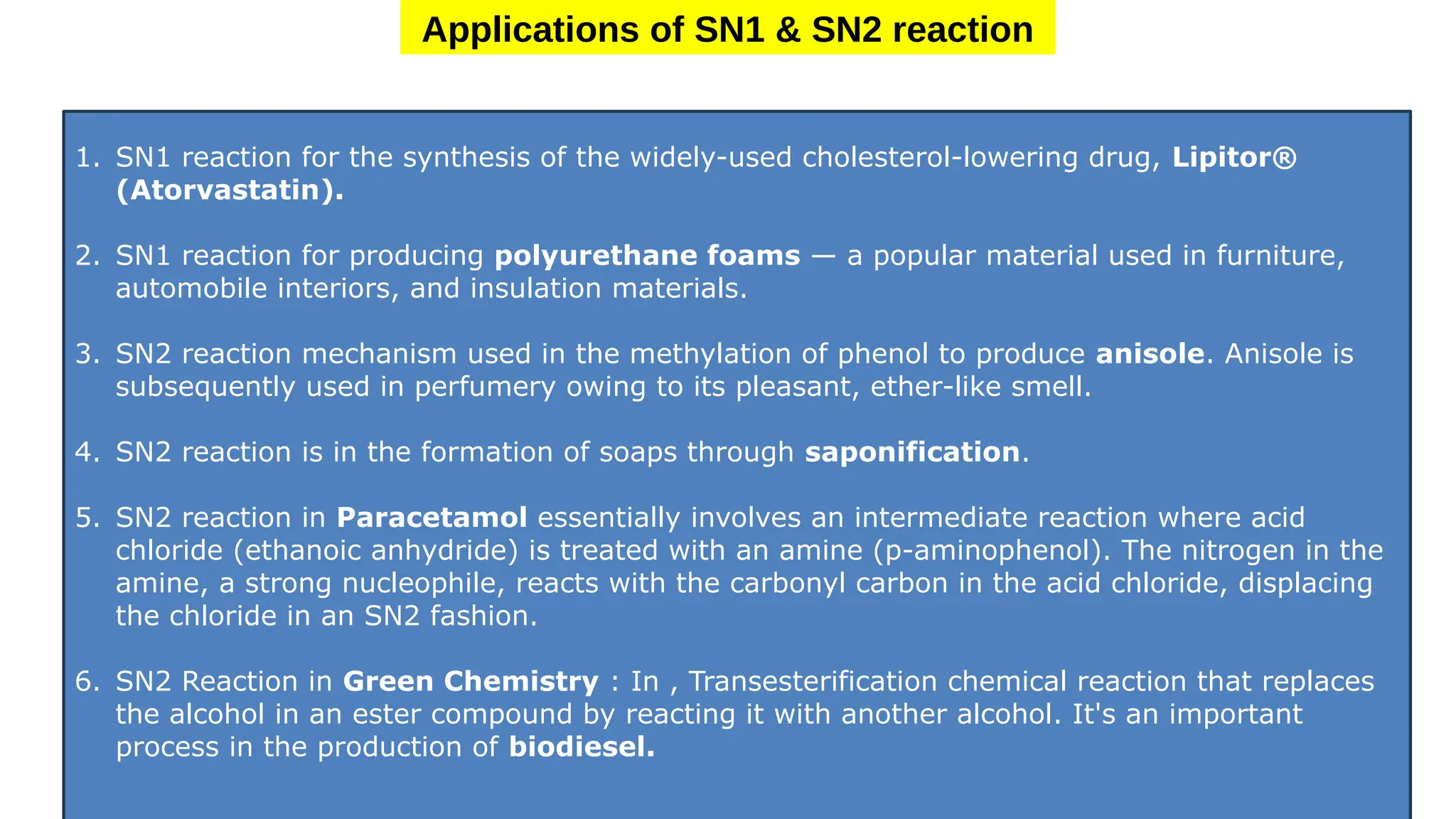
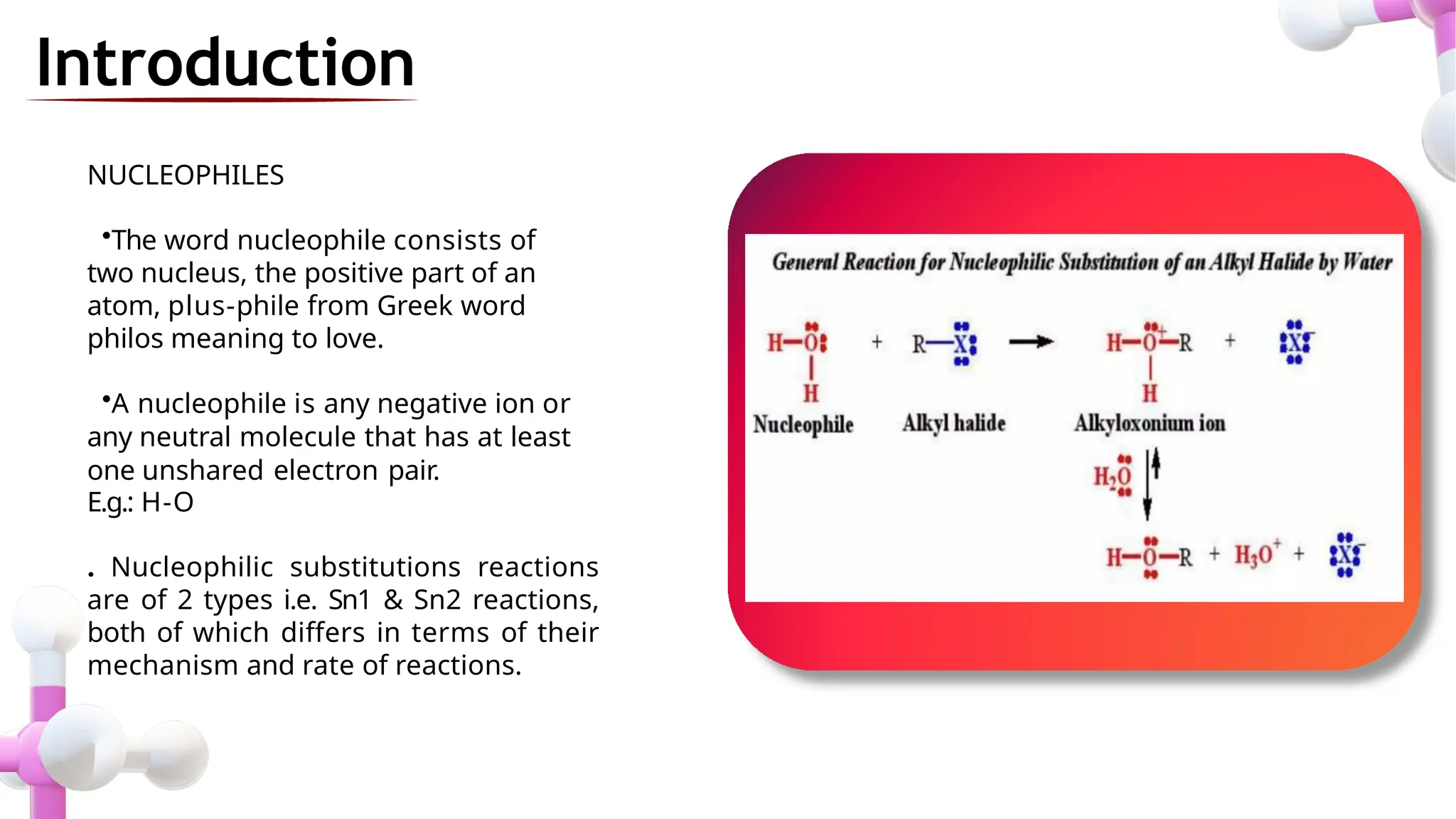
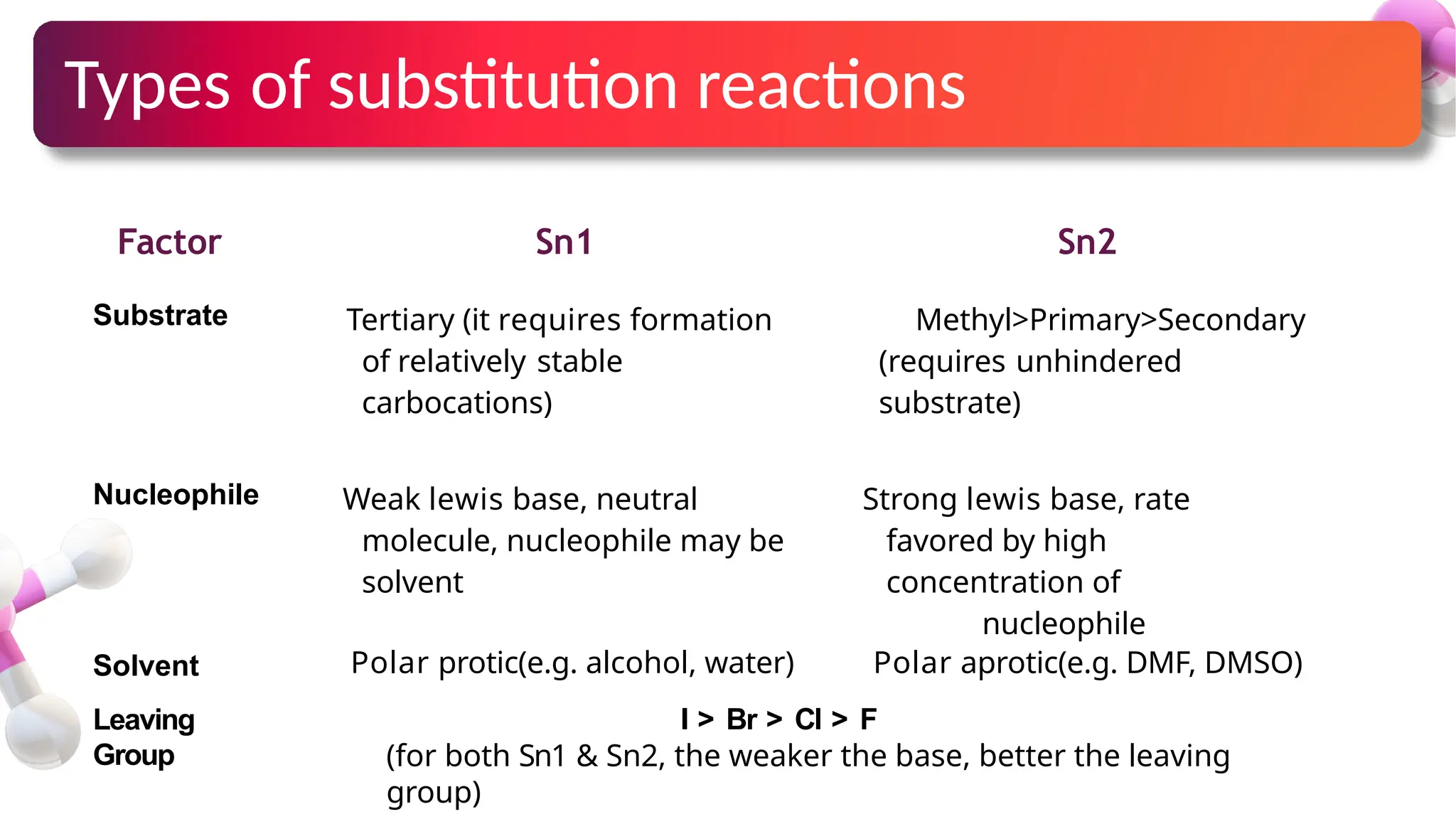
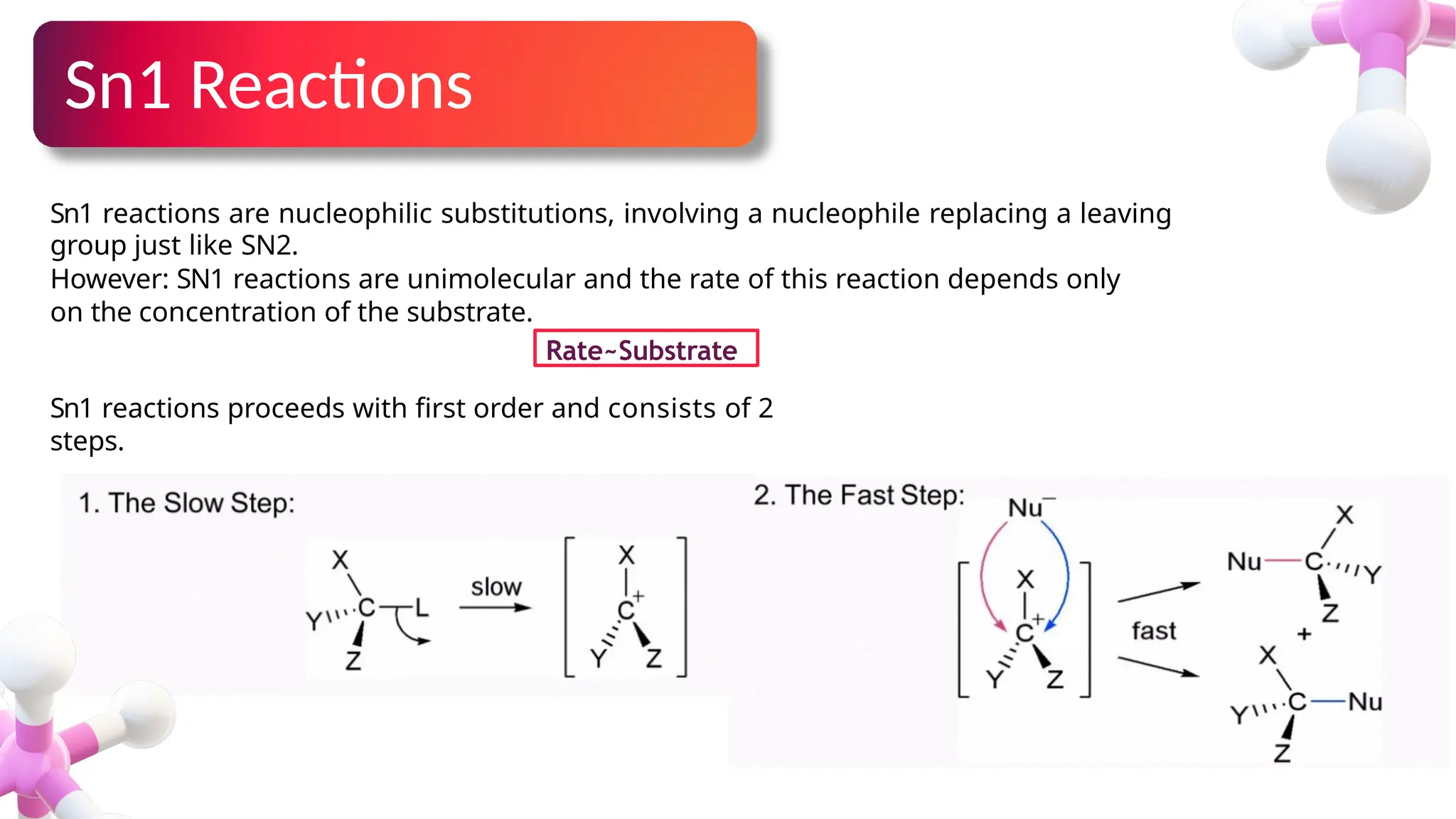
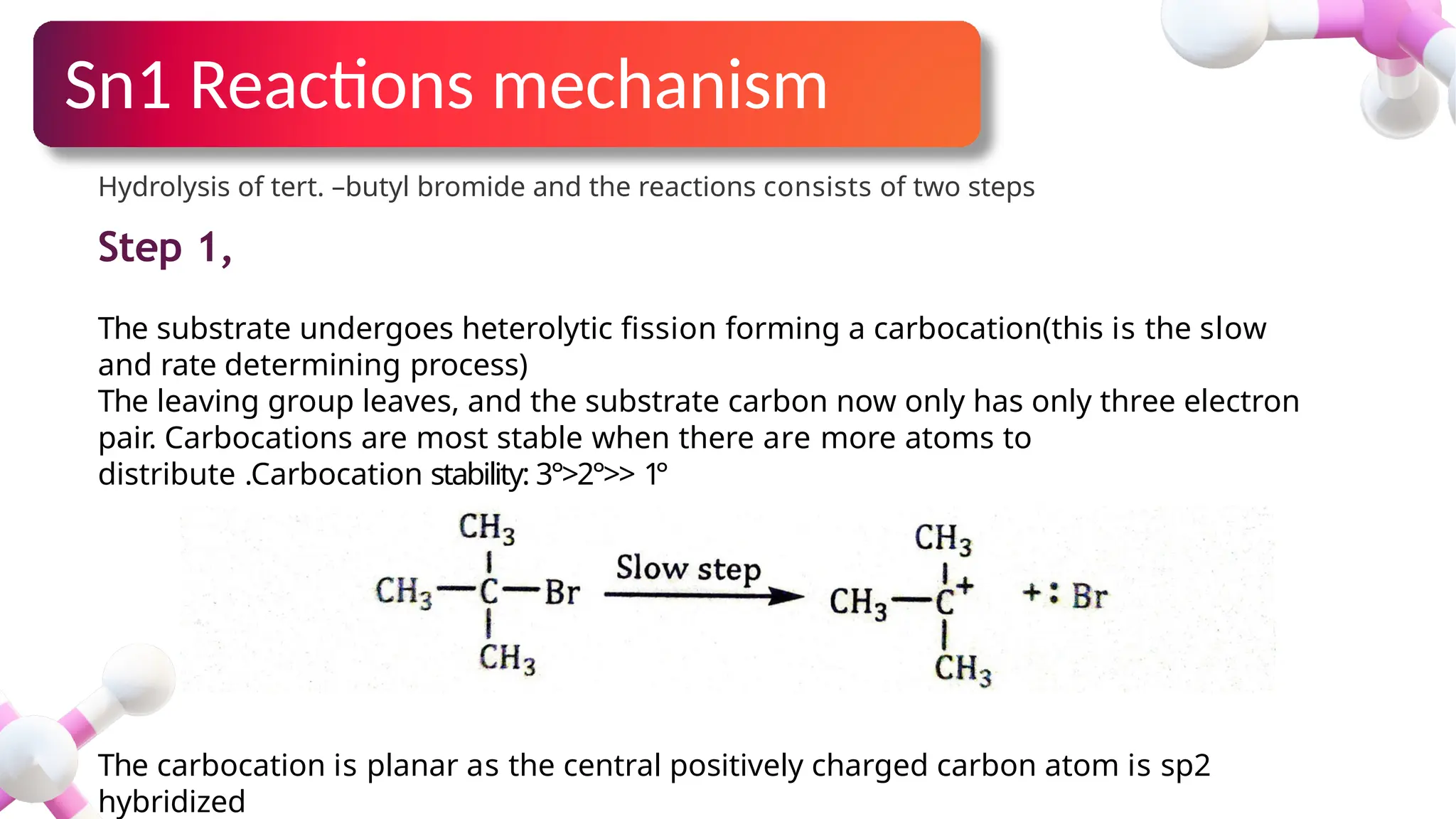
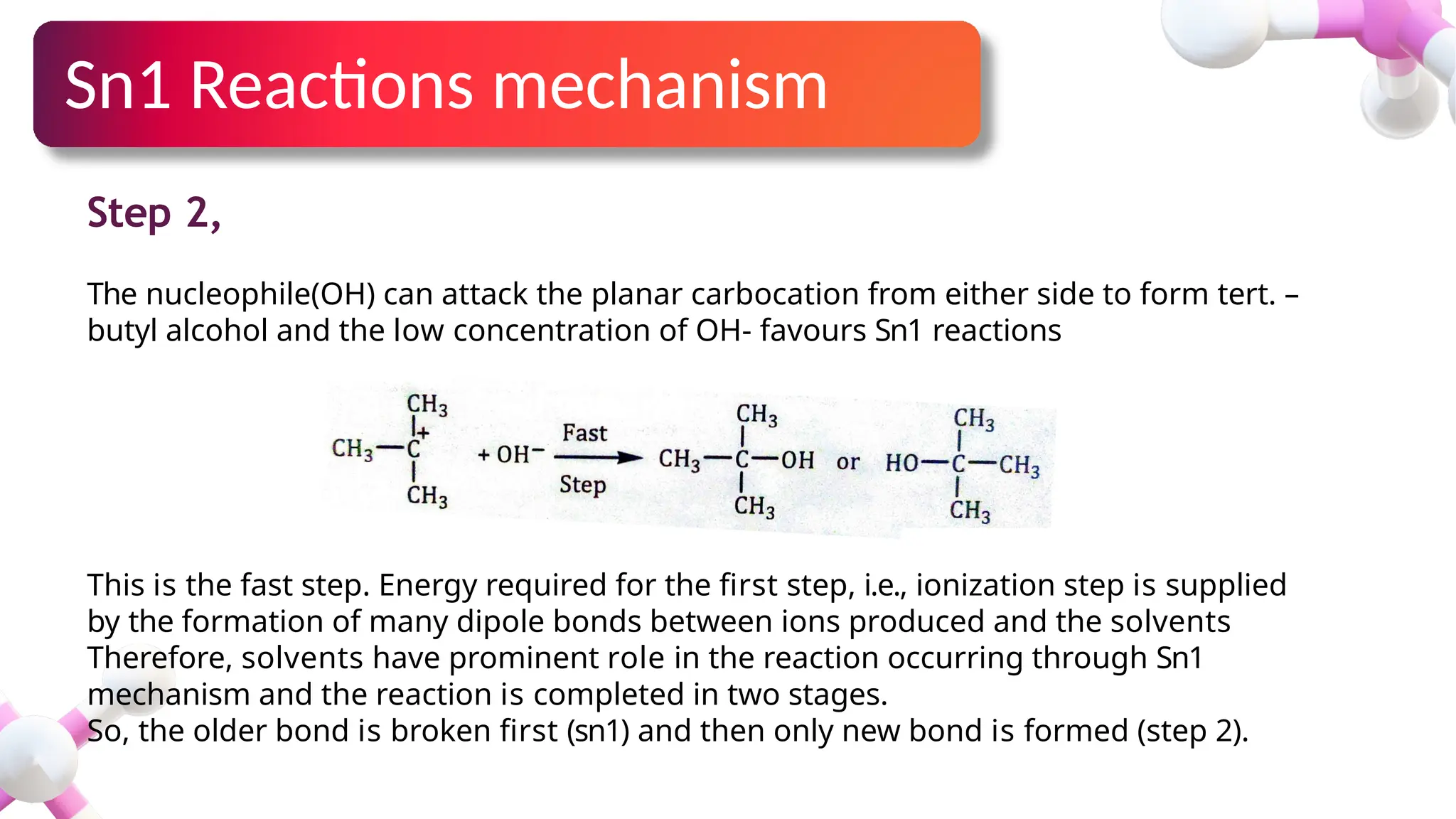
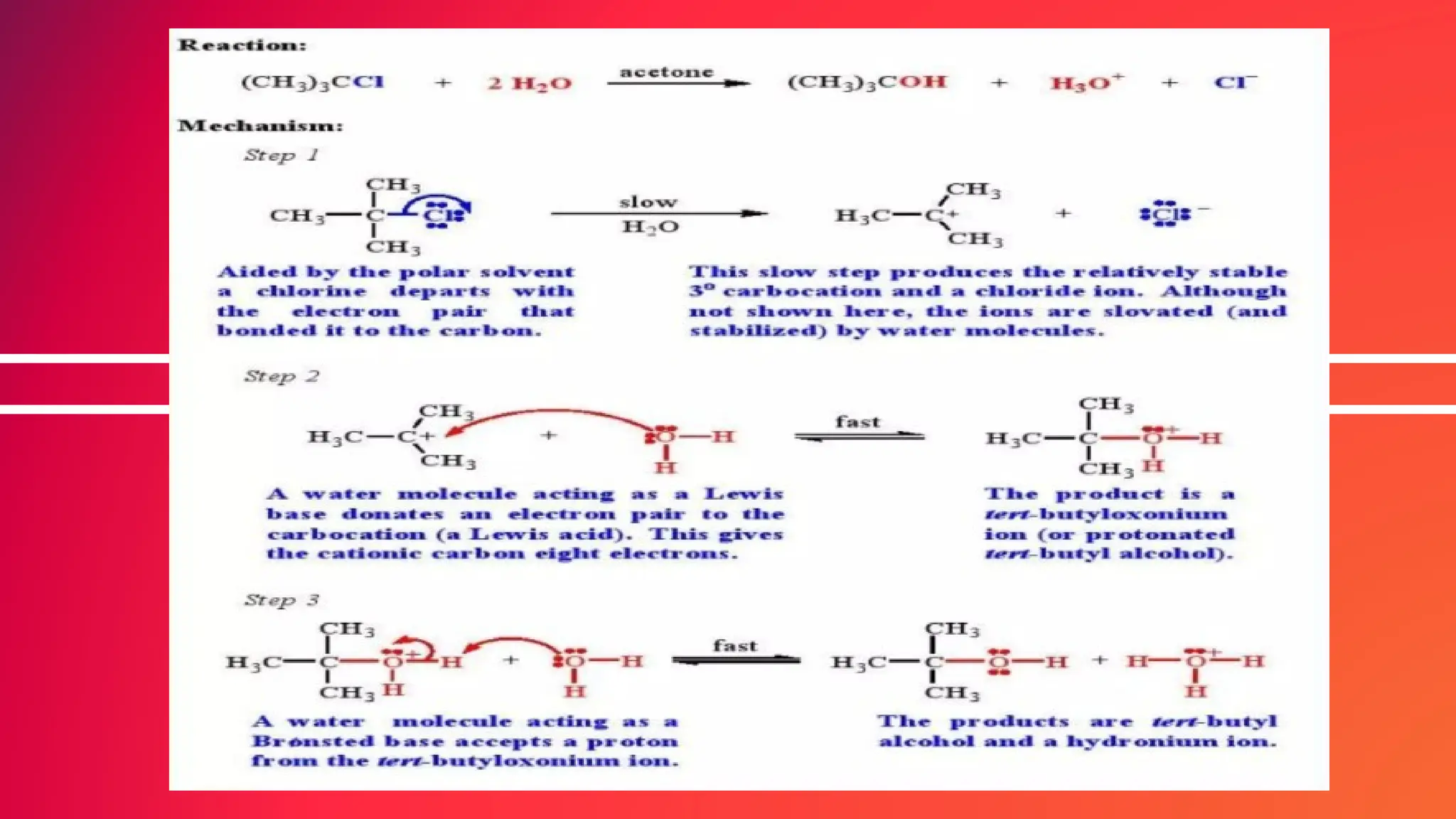
The document provides an overview of nucleophilic substitution reactions, specifically focusing on SN1 and SN2 mechanisms, their characteristics, and differences. It describes the step-by-step processes for each type, highlighting factors that affect reaction rates such as substrate type, nucleophile strength, and steric hindrance. Additionally, it presents various applications of SN1 and SN2 reactions, along with references for further reading.



![The SN2 reaction is a Substitution, Nucleophilic, bimolecular reaction. The transition state involves
both the nucleophile and the substrate, it accounts for the observed second- order reaction rate.
• The SN2 reaction usually involves the loss of a leaving group, which can be a halogen,
nitroxide, alkoxide, or aryloxide group.
• The reaction is catalyzed by bases and proceeds through a concerted mechanism. SN2
reactions are commonly used in the synthesis of new compounds and can be controlled by the
selection of appropriate reaction conditions.
• The reaction is a exothermic reaction where the free energy value G is negative
• Mechanism for SN2 reaction was proposed by Edward D. Hughes and Sir Christopher Ingold (the
University College, London) in 1937.
Example:-
Sn2 Reactions
Rate~[Substrate] [Nucleophile]](https://image.slidesharecdn.com/mpc102tmasn1sn2reactions-241221164433-4b12dfd7/75/Sn1-Sn2-Reactions-and-their-reaction-mechanisms-pptx-10-2048.jpg)