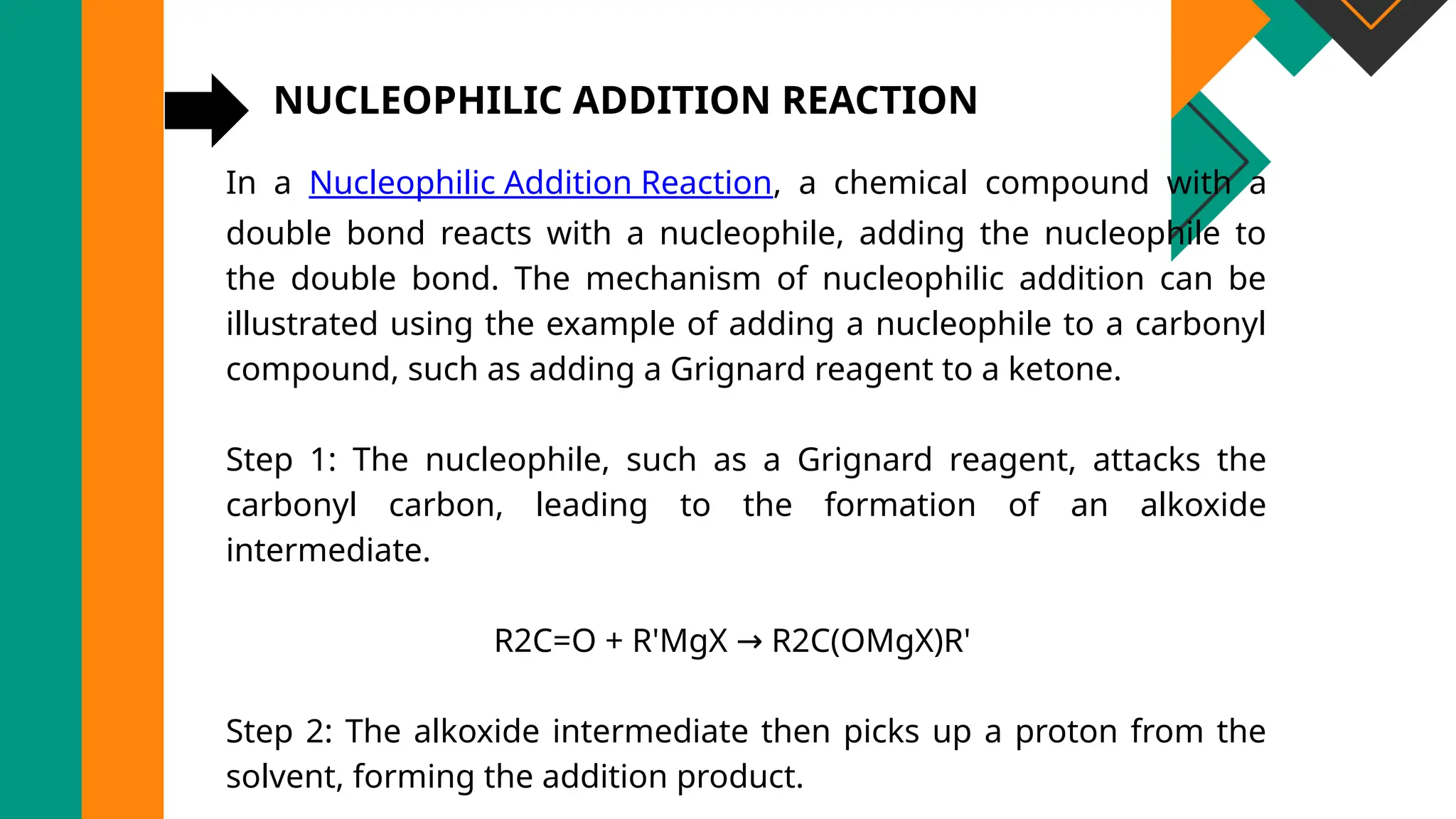
The document discusses electrophilic and nucleophilic addition reactions, explaining their mechanisms, classifications, and applications in organic chemistry. It provides examples such as the hydrogenation of ethene and the addition of hydrogen halides and Grignard reagents. Additionally, it highlights the importance of these reactions in various industries, including pharmaceuticals and plastic production.