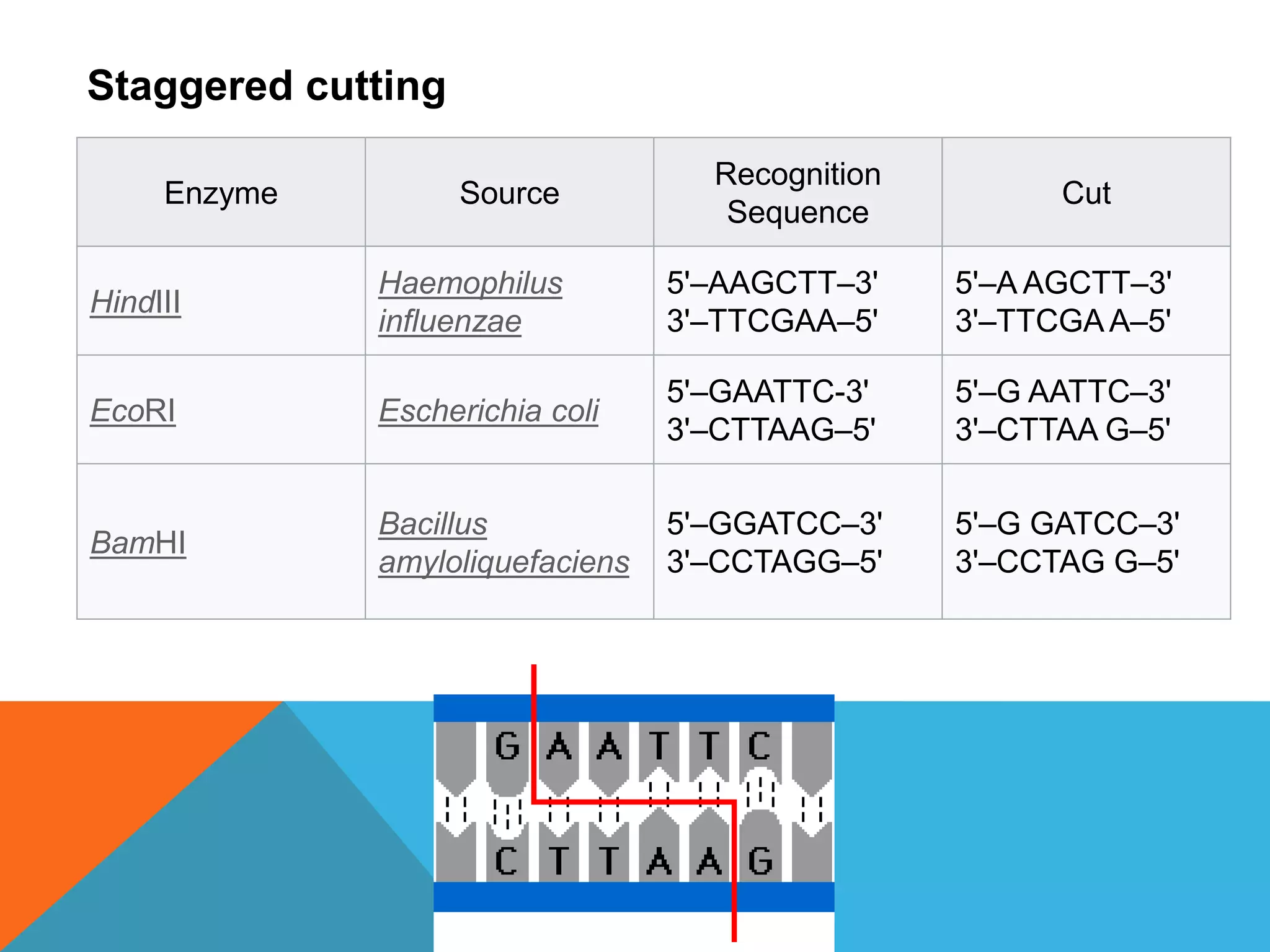
Nucleases are enzymes that cut nucleic acids. There are two main types - endonucleases which cut within strands, and exonucleases which degrade strands from the ends. Restriction endonucleases cut DNA at specific recognition sequences. Key examples are EcoRI, HindIII, and BamHI. Ribonucleases degrade RNA and are divided into endo- and exoribonucleases. RNase A is a common example. Nucleases play important roles in DNA repair and RNA processing through their cleavage activities.