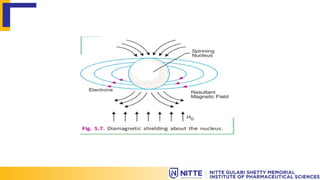
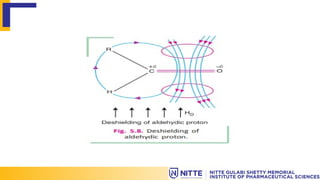
The document explains the principles of Nuclear Magnetic Resonance (NMR) related to shielding and deshielding effects on nuclei in a magnetic field. It describes how electron clouds surrounding nuclei influence magnetic fields, affecting the resonance frequency and resulting chemical shifts in the NMR spectrum. Examples of both shielding and deshielding in organic molecules, such as alkanes and aldehydes, illustrate how structural features and electronegative atoms impact the chemical environment of protons.