The document presents the Nernst equation for calculating the electromotive force (e.m.f) of galvanic cells at non-standard conditions, highlighting its relation to temperature, concentration, and redox reactions. It provides historical context on Walther Hermann Nernst, who developed the equation in 1887 and won the Nobel Prize in 1920. Additionally, the document includes examples and practice problems demonstrating the application of the Nernst equation and calculations of cell potentials and equilibrium constants.

![Nernst Equation
Standard Conditions
C = 1 M
T = 250C (298 K)
Gases = 1 atm
E0
(anode)
E0
(cathode)
E0
cell = [E0
(cathode)] – [E0
(anode)]
The values of E0 can be directly obtained from the
electrochemical series](https://image.slidesharecdn.com/nernstequation3-231009171536-d122516f/75/Nernst-Equation-3-ppt-2-2048.jpg)






![Nernst Equation (contd…)
Given;
E0
Fe
2+
/Fe = -0.44 V (Electrochemical series)
n = 2 and, [Fe2+] = 0.1 M
= -0.44 – (0.0295) = -0.4695 V
Activity: Calculate the electrode potential of
Mg2+/Mg at 250C when the concentration of Mg2+
ions is 0.1M and [Mg 2+]= -2.38
Answer: -2.39V](https://image.slidesharecdn.com/nernstequation3-231009171536-d122516f/75/Nernst-Equation-3-ppt-9-2048.jpg)
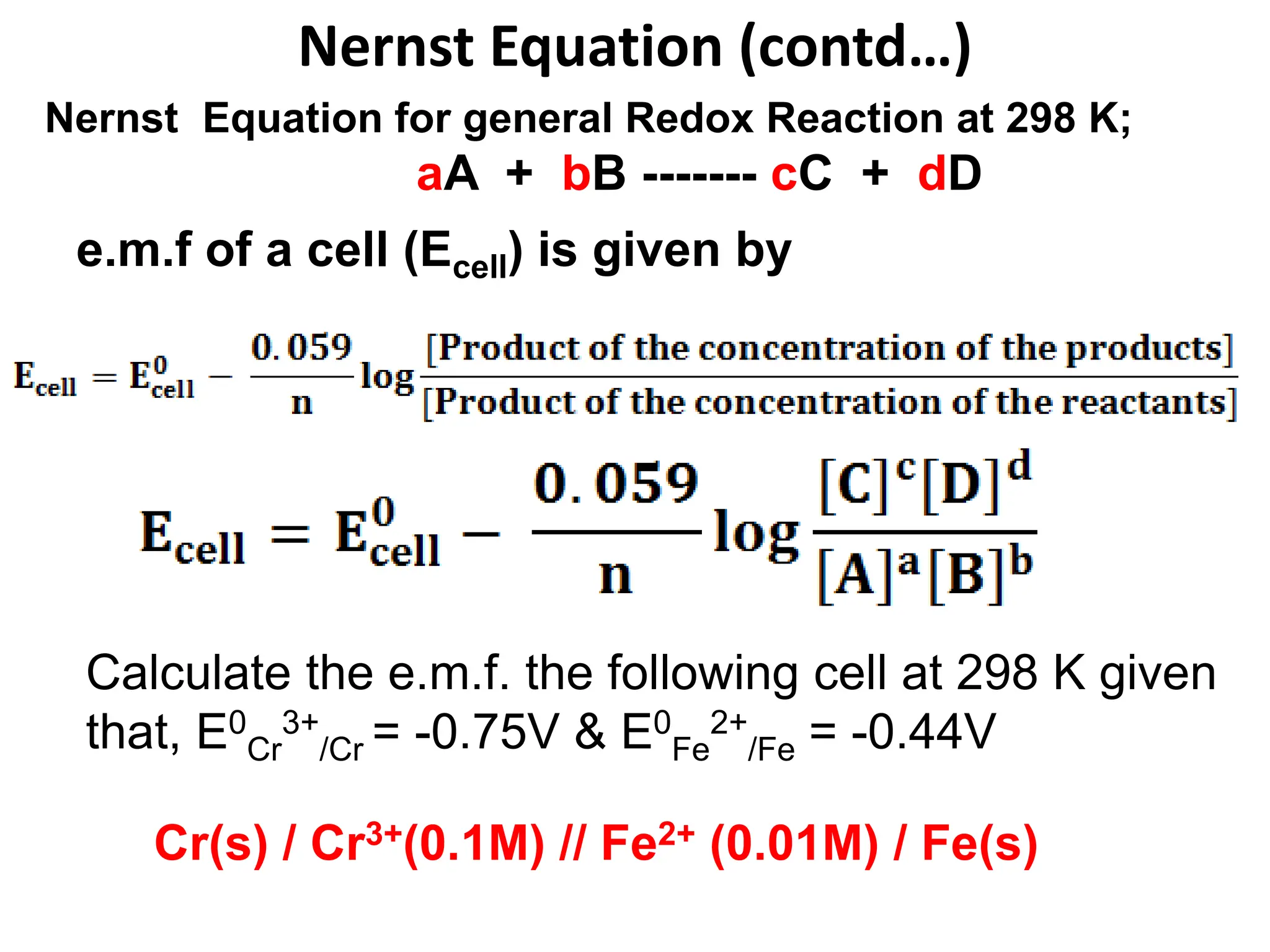
![Nernst Equation (contd…)
Given;
E0
Cr
3+
/Cr = -0.75V (RP) & E0
Fe
2+
/Fe = -0.44V (RP)
[Cr3+] = 0.1M & [Fe2+] = 0.01M
Cell Rep: Cr(s) / Cr3+(0.1M) // Fe2+ (0.01M) / Fe(s)
Cell Reactions
[Cr(s) ----------- Cr3+(0.1M) + 3e- ]
[Fe2+ (0.01M) + 2e- -------- Fe(s) ]
Anode (OHCR)
Cathode (RHCR)
X 2
X 3
2Cr(s) + 3Fe2+(0.01M) -------- 2Cr3+(0.1M) + 3Fe(s) NCR)
n = ??? n = 6
E0
cell = [E0
(cathode)] – [E0
(anode)] = [-0.44] – [-0.75] = 0.31 V](https://image.slidesharecdn.com/nernstequation3-231009171536-d122516f/75/Nernst-Equation-3-ppt-11-2048.jpg)




![Practice Problems
a) Calculate the Kc for the following reaction;
3Sn4+ + 2Cr -------- 3Sn2+ + 2Cr3+
(E0
Sn4+/Sn2+ = +0.15 V & E0
Cr3+/Cr = -0.71V)
Answer:
E0
Cell = 0.15+0.71 = 0.86V, n = 6
Kc = antilog [6 x 0.86]/[0.059] = [5.16]/[0.059] =
1 x 1090
Equilibrium Constant (Kc) of Redox Reaction](https://image.slidesharecdn.com/nernstequation3-231009171536-d122516f/75/Nernst-Equation-3-ppt-16-2048.jpg)



