The document discusses several key concepts in electrochemistry:
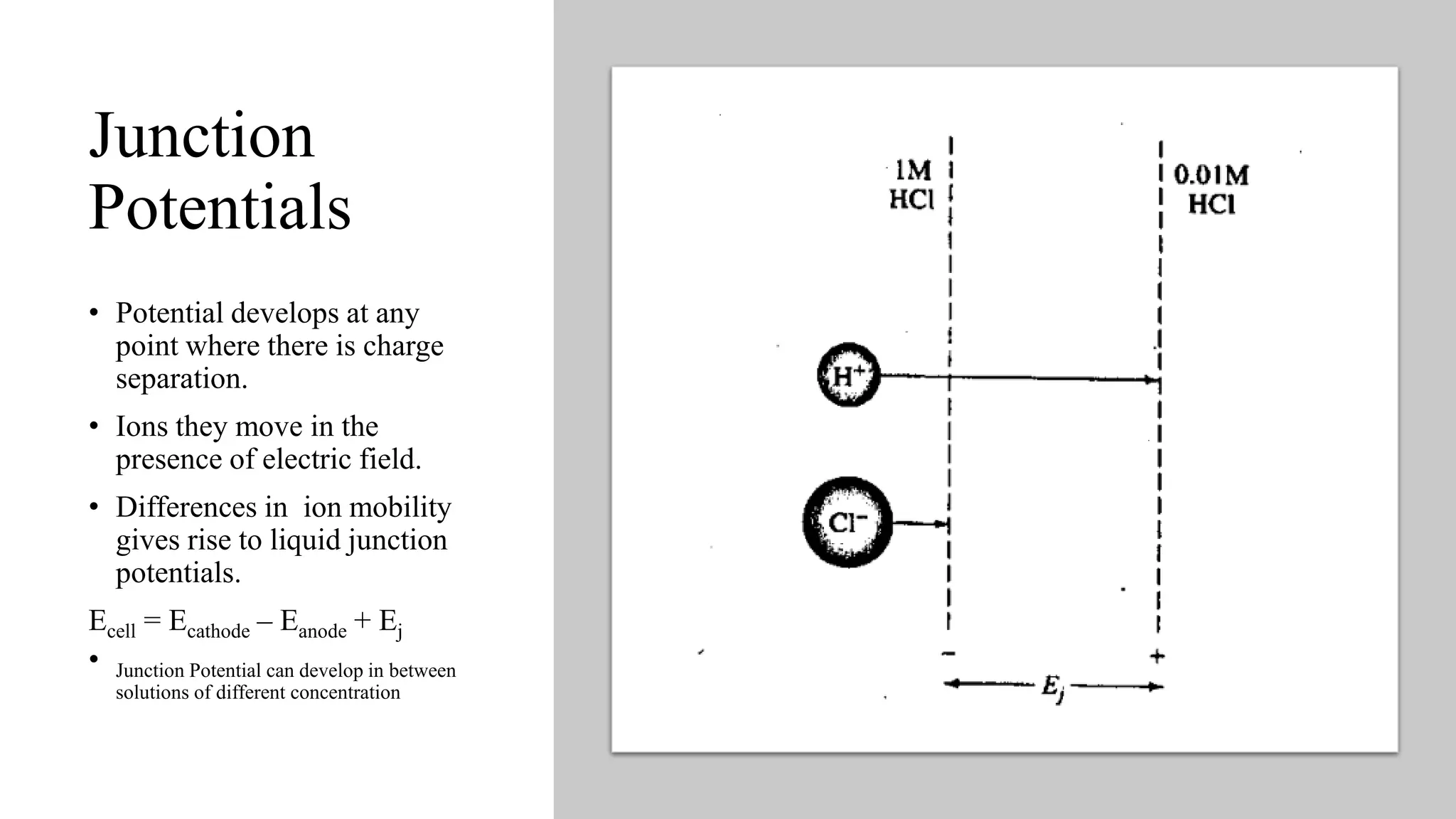
1. Junction potentials develop at any point where there is charge separation between solutions of different concentrations. The difference in ion mobility gives rise to liquid junction potentials.
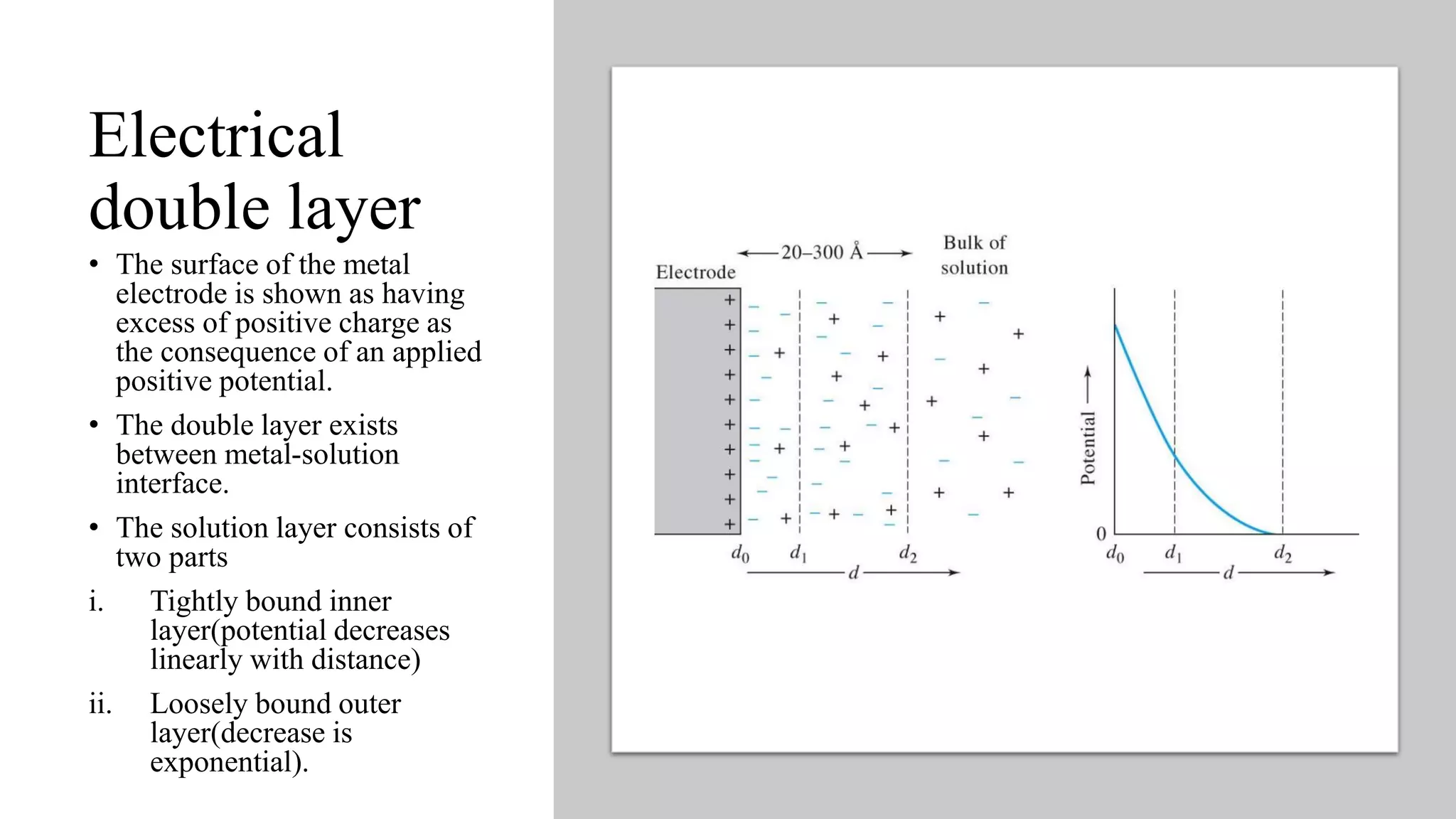
2. At the metal-solution interface, there is an electrical double layer consisting of a tightly bound inner layer and a loosely bound outer layer where the potential decreases exponentially with distance.
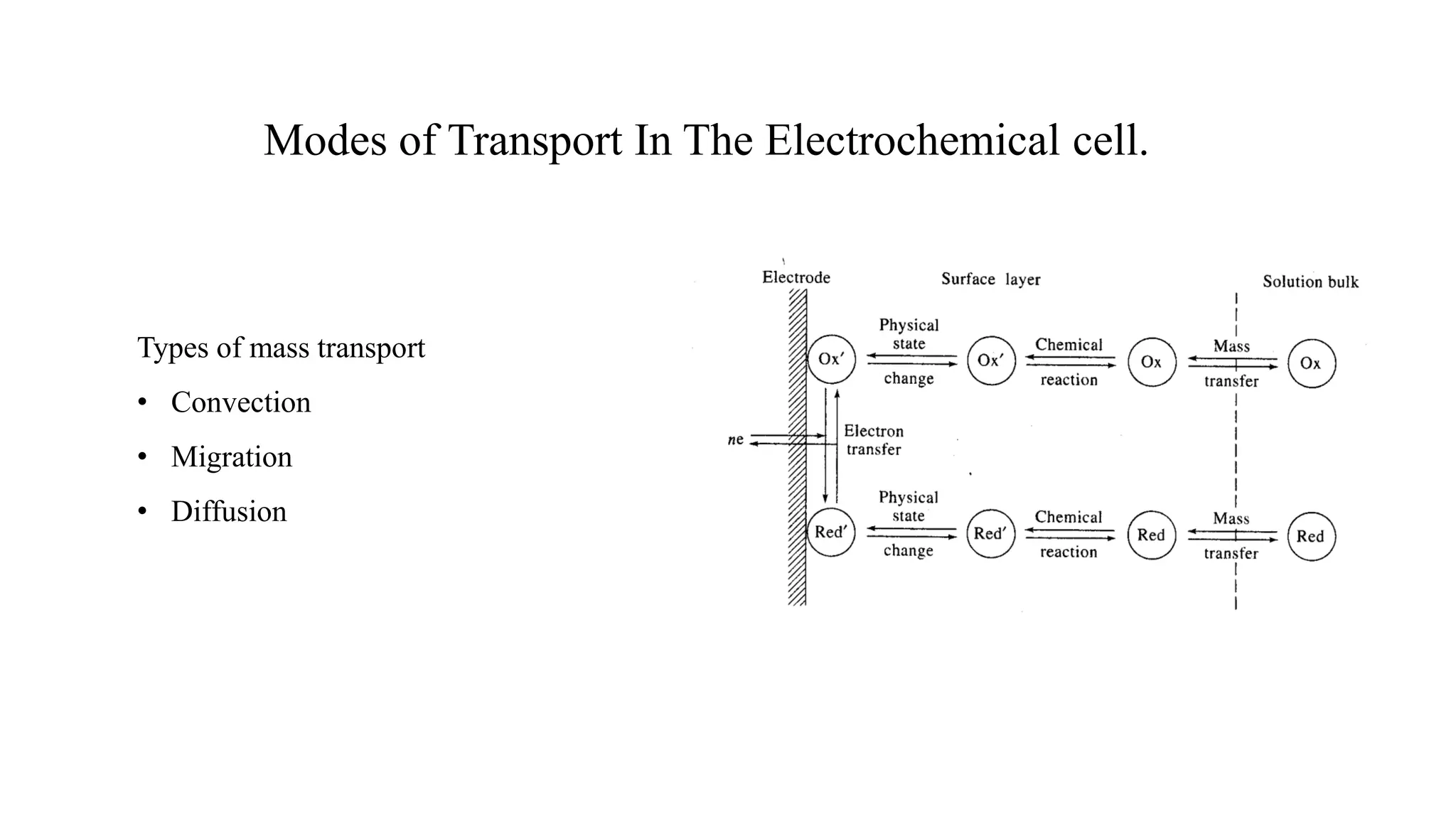
3. Currents in electrochemical cells are limited by charge transfer resistance, mass transport resistance, and ohmic solution resistance. Mass transport occurs via diffusion, convection, and migration. Faradic currents are due to redox reactions while non-Faradic currents are due to other processes.

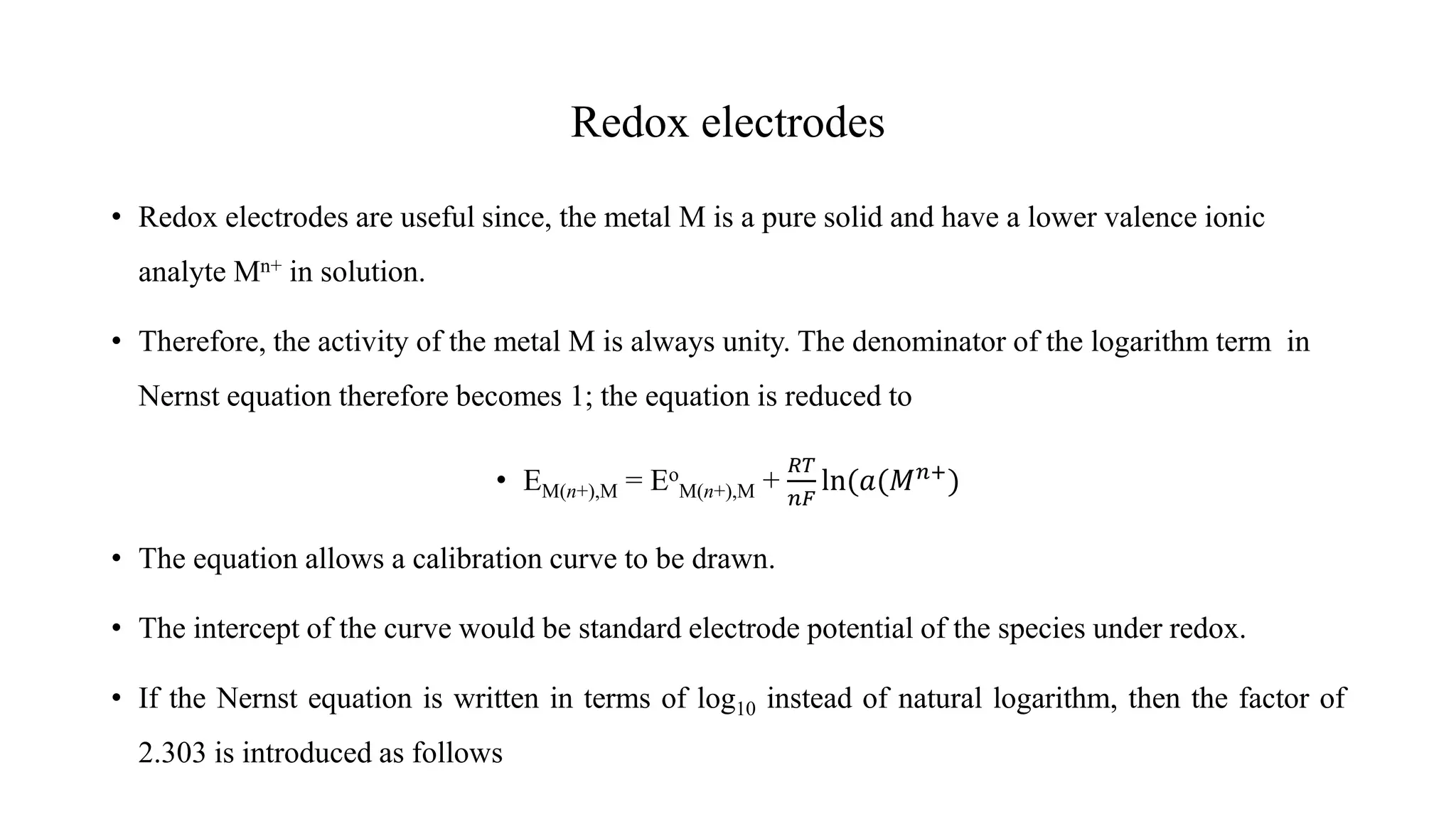
![Nernst equation
• Suppose we have a half-cell reaction
pP + qQ + …ne- → rR + sS + …
• The Nernst equation for the reaction would be
𝐸𝑒𝑙𝑒𝑐𝑡𝑟𝑜𝑑𝑒 = 𝐸𝑜 +
𝑅𝑇
𝑛𝐹
ln
(𝑎𝑅)𝑟
. (𝑎𝑆)𝑠
…
(𝑎𝑝)𝑝. (𝑎𝑄)𝑞 …
Where; R – ideal gas law constant (8.316 J mol-1 K-1), T – absolute temperature (K), F – Faraday
constant, number of electrons involved in a process and a – activity of involved species (γ[X]).](https://image.slidesharecdn.com/lecture5-221104000826-acead563/75/Lecture-5-pdf-7-2048.jpg)
![• Step 1
Emf = SCE - EFe|Fe2+
Therefore, EFe|Fe2+ = -0.472 V
• Step 2
Determining the concentration of [Fe2+] – it is actually activity of aquo Fe (a((Fe2+)).
EFe2+,Fe = Eo
Fe2+, Fe +
𝑅𝑇
𝑛𝐹
ln[
𝑎(𝐹𝑒2+)
𝑎(𝐹𝑒)
]
From standard reduction potential, Eo
Fe2+, Fe = -0.44 V
The unit
𝑅𝑇
𝐹
= (8.316 𝐽 𝑚𝑜𝑙−1
𝐾−1
× 298 𝐾)/(96486 J 𝑉−1
) = 0.0257 V
In the redox couple, Fe is metallic therefore its activity is unity.
Therefore ,
-0.472 V = -0.44 V +
0.0257 V
2
(ln(a(𝐹𝑒2+
))
ln(a(Fe2+) =
2 −0.44 𝑉+0.44 𝑉
0.0257 𝑉
Rearranging, we get
ln(a(𝐹𝑒2+
) = -2.490
a(𝐹𝑒2+
)= exp(-2.490) = 0.0829.](https://image.slidesharecdn.com/lecture5-221104000826-acead563/75/Lecture-5-pdf-9-2048.jpg)

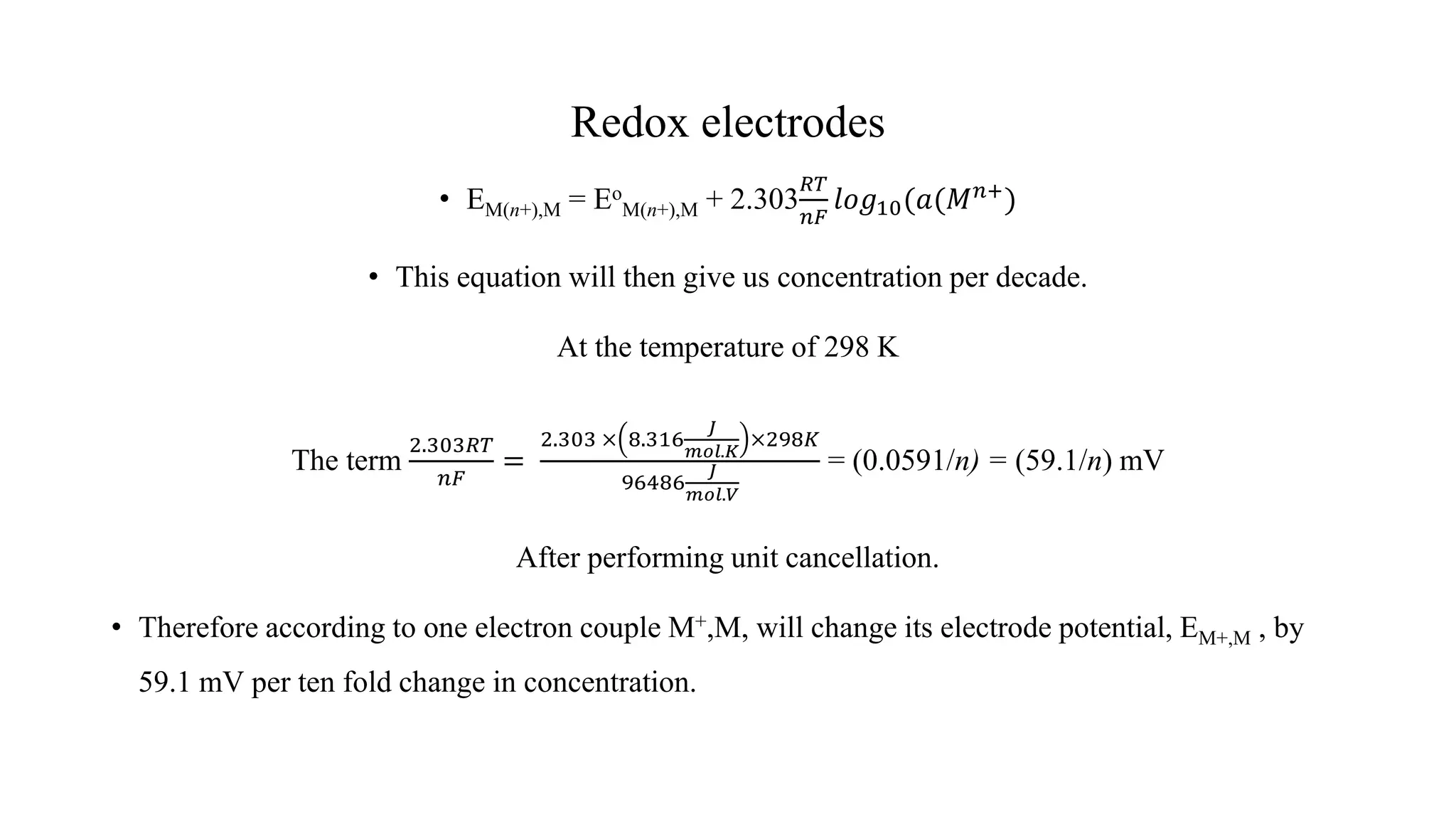
![Redox Electrodes
• Note that
59.1
𝑛
𝑚𝑉 𝑖𝑠 𝑡ℎ𝑒 𝑠𝑙𝑜𝑝𝑒 𝑜𝑓 𝑡ℎ𝑒 𝑁𝑒𝑟𝑛𝑠𝑡 𝑒𝑞𝑢𝑎𝑡𝑖𝑜𝑛 𝑤ℎ𝑒𝑛 𝑙𝑜𝑔10 is used to the equation.
• The logarithm base 10 gives us number of decades of concentration.
• Then the Nernst equation reduces to
• EMn+,M = Eo
Mn+, M – [number of decades of dilution × 59.1
𝑚𝑉
𝑛
]
• The resultant equation is an approximate of Nernst equation.
• Example: A new way of extracting Nickle from its ore ore is been investigated. The first step is to crash
the rock powder, roast it, and then extract soluble Ni2+ species in aqueous solution. The activity of nickel
a(Ni2+) is monitored by potentiometric method, where a wire of pure nickel metal functions as an
electrode and is immersed in aliquot samples taken from the plant. The wire monitors the electrode
potential ENi2+,N . If Eo
Ni2+,Ni = -0.230 V, what is the ENi2+,N if a(Ni2+) = 10-6.](https://image.slidesharecdn.com/lecture5-221104000826-acead563/75/Lecture-5-pdf-14-2048.jpg)