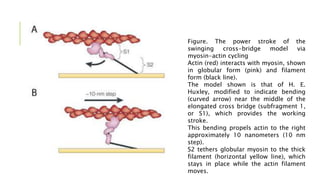
- Muscle contraction occurs via the sliding filament theory where actin filaments slide past myosin filaments, causing sarcomeres and muscles to shorten.
- Sarcomeres contain regularly arranged thick and thin filaments that generate striations visible under a microscope. Contraction is driven by myosin cross-bridges binding to actin and generating a power stroke via ATP hydrolysis.
- Calcium binds to troponin and causes it to shift tropomyosin, unblocking the myosin binding sites on actin and allowing cross-bridge cycling and contraction to occur.